Synthesis and urea adsorption capacity of a strong, acidic hollow nanoparticle
IF 2.3
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
Abstract
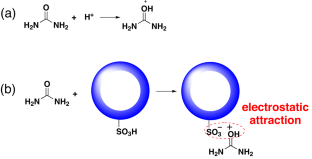
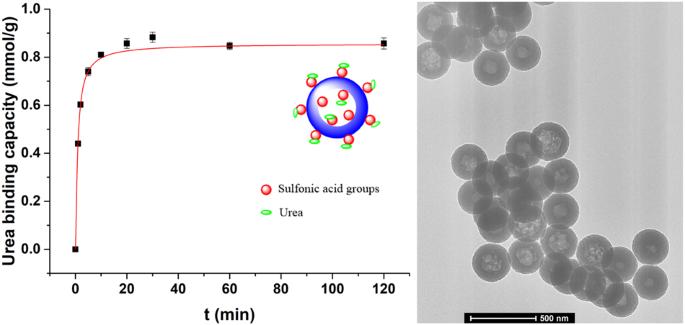
To increase the quality of life of dialysis patients while maintaining economic efficiency, the concept of a wearable artificial kidney was proposed and designed approximately two decades ago. However, the primary challenge in the development of a wearable artificial kidney is the adequate removal of urea from dialysate due to the chemical inertness of urea under physiological conditions. Herein, a hollow polystyrene nanoparticle with sulfonic acid groups, named H-CPS-SO3H, was synthesized that could efficiently adsorb urea. H-CPS-SO3H was produced in three steps. First, a core-shell polystyrene nanoparticle with a linear core and cross-linked shell was prepared using modified emulsion polymerization. Second, the core-shell nanoparticles were treated with DMF to create hollow nanoparticles. Finally, the hollow nanoparticles were subjected to sulfuric acid treatment to produce H-CPS-SO3H, which was confirmed by both TEM and FTIR analysis. The urea adsorption capacity and kinetics of the as-synthesized H-CPS-SO3H were evaluated in a 30 mM urea aqueous solution. The results indicated that H-CPS-SO3H had a urea absorption capacity of up to 1 mmol/g, which was achieved after only two hours of adsorption at 37 °C. These findings demonstrated the high adsorption capacity and favorable adsorption kinetics of H-CPS-SO3H. Additionally, the adsorption capacity first increased and then slightly decreased with decreasing pH or increasing solution volume, while the adsorption capacity sharply decreased with increasing ionic strength. The results suggest that the prepared H-CPS-SO3H has promising application potential in the field of wearable artificial kidney devices. Achieving a wearable artificial kidney hinges on overcoming the critical challenge of developing efficient urea adsorption materials for dialysate regeneration. An acidic hollow polystyrene nanoparticle was synthesized by modified emulsion polymerization, DMF etching and sulfuric acid treatment sequentially. The nanoparticles had a urea absorption capacity of up to 1 mmol/g after two hours of adsorption in a 30 mM urea aqueous solution at 37 °C. Additionally, the adsorption capacity dramatically increased with increasing urea concentration, while sharply decreased with increasing ionic strength.
强酸性中空纳米粒子的合成与尿素吸附能力
为了提高透析患者的生活质量,同时保持经济效益,大约二十年前,人们提出并设计了可穿戴式人工肾脏的概念。然而,由于尿素在生理条件下的化学惰性,开发可穿戴式人工肾脏的首要挑战是如何充分去除透析液中的尿素。在此,我们合成了一种带有磺酸基团的中空聚苯乙烯纳米粒子,命名为 H-CPS-SO3H,它能有效地吸附尿素。H-CPS-SO3H 分三步制得。首先,利用改性乳液聚合法制备出具有线性内核和交联外壳的核壳聚苯乙烯纳米粒子。其次,用 DMF 处理核壳纳米粒子,生成空心纳米粒子。最后,将中空纳米粒子进行硫酸处理,生成 H-CPS-SO3H,并通过 TEM 和 FTIR 分析加以确认。在 30 mM 尿素水溶液中对合成的 H-CPS-SO3H 的尿素吸附能力和动力学进行了评估。结果表明,H-CPS-SO3H 的尿素吸附能力高达 1 mmol/g,而且在 37 °C 下仅吸附两小时就达到了这一吸附能力。这些结果表明 H-CPS-SO3H 具有很高的吸附容量和良好的吸附动力学。此外,随着 pH 值的降低或溶液体积的增加,吸附容量先增加后略有下降,而随着离子强度的增加,吸附容量急剧下降。结果表明,制备的 H-CPS-SO3H 在可穿戴人工肾设备领域具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


