Exosomal miR-126-3p: Potential protection against vascular damage by regulating the SLC7A5/mTOR Signalling pathway in human umbilical vein endothelial cells
IF 4.1
4区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
Systemic sclerosis (SSc) is a chronic autoimmune connective tissue disease. Vascular damage is one of the important features of SSc, which affects the progression and prognosis of the disease. MiR-126-3p is an important microRNA (miRNA) that regulates vascular structure and function, which can be transported through exosomes. However, the role of miR-126-3p in vascular damage in SSc is still unclear. Therefore, we focused on the connection between miR-126-3p and vascular damage in SSc, as well as investigated the potential role of miR-126-3p in vascular damage in SSc. First, this study successfully extracted extracellular vesicles from clinical plasma samples and characterized the exosomes within them. Then, we predicted and screened the target pathway mammalian/mechanistic target of rapamycin (mTOR) and the target gene SLC7A5 of miR-126-3p through online databases. Next, we constructed SSc mice for in vivo studies. The results showed that the expression of miR-126-3p was decreased in the plasma exosomes, while the SLC7A5 expression, autophagy, and lipid peroxidation were increased in the aorta. Luciferase reporter gene assays demonstrated that miR-126-3p can bind to SLC7A5, resulting in a decrease in its expression. In vitro experiments have shown that exosomal miR-126-3p can be internalized by human umbilical vein endothelial cells (HUVECs). The miR-126-3p group exhibited enhanced cell viability and tube formation ability, along with increased expression of the vascular formation marker CD31. Additionally, miR-126-3p downregulated the protein expression of SLC7A5 and LC3 in HUVECs, while upregulating the protein expression of mTOR, P62, PPARγ, and CPT-1. However, the effects of miR-126-3p on HUVECs were counteracted by mTOR inhibitors and enhanced by mTOR activators. The results indicated that exosomal miR-126-3p has the potential to protect against vascular injury in SSc by regulating the SLC7A5/mTOR signalling pathway in HUVECs.
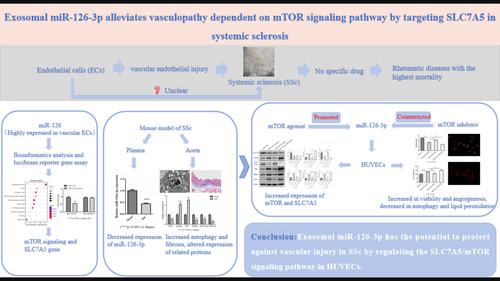
外泌体 miR-126-3p:通过调节人脐静脉内皮细胞中的 SLC7A5/mTOR 信号通路来防止血管损伤的潜力
系统性硬化症(SSc)是一种慢性自身免疫性结缔组织疾病。血管损伤是系统性硬化症的重要特征之一,会影响疾病的进展和预后。MiR-126-3p 是一种调节血管结构和功能的重要微RNA(miRNA),可通过外泌体运输。然而,miR-126-3p 在 SSc 血管损伤中的作用仍不清楚。因此,我们重点研究了 miR-126-3p 与 SSc 血管损伤之间的联系,并探讨了 miR-126-3p 在 SSc 血管损伤中的潜在作用。首先,本研究成功地从临床血浆样本中提取了细胞外囊泡,并对其中的外泌体进行了表征。然后,我们通过在线数据库预测和筛选了miR-126-3p的靶途径哺乳动物/雷帕霉素机械靶标(mTOR)和靶基因SLC7A5。接下来,我们构建了 SSc 小鼠进行体内研究。结果表明,血浆外泌体中 miR-126-3p 的表达量减少,而主动脉中 SLC7A5 的表达量、自噬和脂质过氧化增加。荧光素酶报告基因实验证明,miR-126-3p 可与 SLC7A5 结合,导致其表达量减少。体外实验表明,外泌体 miR-126-3p 可被人脐静脉内皮细胞(HUVECs)内化。miR-126-3p 组的细胞活力和管形成能力增强,血管形成标志物 CD31 的表达也增加。此外,miR-126-3p 下调了 HUVECs 中 SLC7A5 和 LC3 的蛋白表达,同时上调了 mTOR、P62、PPARγ 和 CPT-1 的蛋白表达。然而,miR-126-3p 对 HUVEC 的影响会被 mTOR 抑制剂抵消,而被 mTOR 激活剂增强。结果表明,外泌体 miR-126-3p 有可能通过调节 HUVECs 中的 SLC7A5/mTOR 信号通路来保护 SSc 血管免受损伤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
5.40%
发文量
109
审稿时长
1 months
期刊介绍:
This peer-reviewed international journal publishes original articles and reviews on all aspects of basic, translational and clinical immunology. The journal aims to provide high quality service to authors, and high quality articles for readers.
The journal accepts for publication material from investigators all over the world, which makes a significant contribution to basic, translational and clinical immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


