Continuous flow ozonolysis of cardanol for greener synthesis of bio-based monomers
Abstract
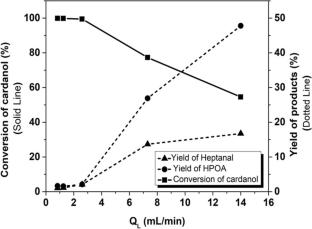
Synthesis of bio-based monomers via continuous flow ozonolysis of cardanol using a simple tubular reactor is demonstrated. The direct ozonolysis of cardanol produces unique monomer 8-(3-hydroxyphenyl) octanal (HPOA) and heptanal along with several other oxidation products. Maximum 47% yield of HPOA with 54.3% conversion of cardanol was obtained at 0 °C in 9 s. The complete conversion of cardanol was obtained at the ozone to cardanol molar flow ratios greater than 2 at all temperatures varied in the range of -10 °C to 20 °C. Owing to large gas–liquid ratios, the mass transfer limitation for transfer of ozone from gas to liquid was negligible; however, the extent of axial dispersion in the liquid phase was significant at lower liquid flow rates. The non-ideal behavior was incorporated in the axial dispersion model to predict the conversion of cardanol. Examination of kinetic rates by both ideal plug-flow model and plug-flow with axial dispersion model revealed that the reaction is fast and is least influenced by the axial-dispersion in the reactor at prevailing operating conditions. The findings of the current study show that continuous flow technique enables a simple and safer synthesis of high-value bio-based monomers via ozonolysis of cardanol compared to traditional batch methods.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


