Enantioselective synthesis of β- and α-amino ketones through reversible alkane carbonylation
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The direct incorporation of alkanes and CO into value-added chiral products through alkane carbonylation is a desirable transformation; however, it remains inefficient. The carbonylation of alkanes via photoirradiated radical addition to CO requires mild reaction conditions but suffers from low conversion due to equilibrium constraints. Here an equilibrium-leveraging strategy that combines alkane carbonylation with various enantioselective transformations is reported. The combination of tetra-n-butylammonium decatungstate and chiral sodium phosphate catalysts enables alkane carbonylation/enantioselective Mannich reaction and alkane carbonylation/enantioselective radical addition cascade processes for the enantioselective synthesis of β-amino and α-amino ketones from alkanes, CO and anilines by breaking the equilibrium of reversible photocatalytic C–H carbonylation. While both reactions can tolerate a broad scope of cyclic alkanes and anilines, the synthetic method to synthesize β-amino ketones can use a range of aliphatic ketones as substrates. The synthetic process to form β-amino ketones can be readily scaled-up through use of an integrated continuous-flow and batch set-up, providing efficient gram-scale synthesis. Mechanistic studies reveal that the synthesis of α-amino ketones proceeds through the asymmetric addition of an acyl radical to an imine intermediate. Alkane carbonylation through photocatalytic alkyl radical addition to CO is a challenge. Now, an equilibrium-leveraging strategy which combines the direct carbonylation of alkanes with CO with onwards enantioselective transformations is reported, providing an enantioselective method for the synthesis of β-amino and α-amino ketones.
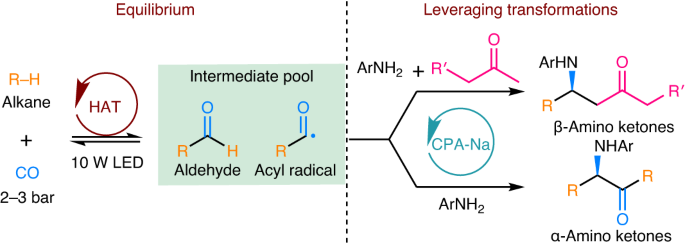
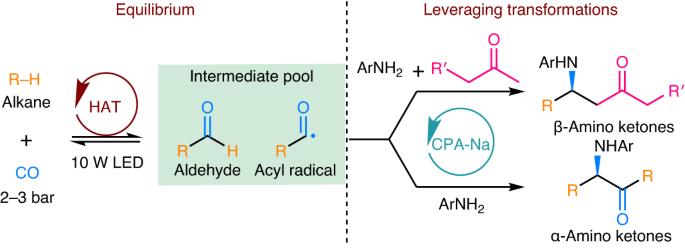
通过可逆烷烃羰基化技术对β-和α-氨基酮进行对映选择性合成
通过烷烃羰基化将烷烃和一氧化碳直接转化为高附加值的手性产品是一种理想的转化方法,但其效率仍然很低。通过光辐射自由基加成 CO 进行烷烃羰基化需要温和的反应条件,但由于平衡限制,转化率较低。本文报告了一种将烷烃羰基化与各种对映选择性转化相结合的平衡杠杆策略。将癸钨酸四正丁基铵和手性磷酸钠催化剂结合在一起,通过打破可逆光催化 C-H 羰基化的平衡,实现了烷烃羰基化/对映体选择性曼尼希反应和烷烃羰基化/对映体选择性自由基加成级联过程,从而从烷烃、CO 和苯胺中对映体选择性地合成了 β-氨基和 α-氨基酮。虽然这两种反应都可以耐受范围广泛的环烷和苯胺,但合成 β-氨基酮的合成方法可以使用一系列脂肪族酮作为底物。形成 β-氨基酮的合成工艺可通过使用集成的连续流和间歇装置方便地进行放大,从而提供高效的克级合成。机理研究表明,α-氨基酮的合成是通过酰基与亚胺中间体的不对称加成反应进行的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


