Printable Single-Ion Polymer Nanoparticle Electrolytes for Lithium Batteries
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
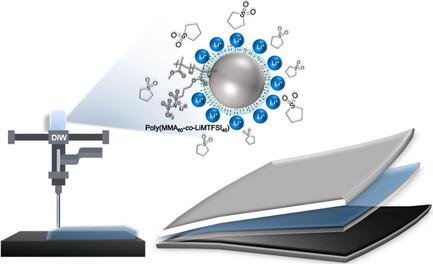
New material solutions are searched for the manufacturing and safety of current batteries. Herein, an extrusion printable polymer separator for lithium batteries based on single-ion polymer electrolytes is presented. The polymer electrolytes are based on methacrylic polymeric nanoparticles (NPs) functionalized with a lithium sulfonamide group combined with different organic plasticizers such as sulfolane and carbonates. The synthesis of the polymer NPs is carried out by emulsion copolymerization of methyl methacrylate and lithium sulfonamide methacrylate in the presence of a crosslinker, resulting in particle sizes of less than 30 nm, as shown by electron microscopy. Then polymer electrolytes are prepared by mixing polymer NPs with varying lithium sulfonamide content and different plasticizers such as carbonates and sulfolane. The polymer electrolytes show ionic conductivities between 2.9 × 10−4 and 2.3 × 10−5 S cm−1 at 85 °C with the highest values for the small-sized NPs with the highest lithium content. As a proof-of-concept application, layer-by-layer printing of a sulfolane-based polymer electrolyte is evaluated via direct ink writing directly onto classic battery electrodes. The electrochemical characterization of the printed solid electrolyte indicates favorable properties, ionic conductivity, lithium transfer number, electrochemical stability window, and cyclability in lithium symmetrical cells, to be used in lithium batteries.
用于锂电池的可打印单离子聚合物纳米粒子电解质
为了提高当前电池的制造和安全性,人们一直在寻找新的材料解决方案。本文介绍了一种基于单离子聚合物电解质的可挤压打印锂电池聚合物隔膜。这种聚合物电解质以甲基丙烯酸聚合物纳米颗粒(NPs)为基础,该纳米颗粒由磺酰胺锂基团与不同的有机增塑剂(如磺烷和碳酸盐)组合而成。聚合物 NPs 的合成是通过甲基丙烯酸甲酯和甲基丙烯酸磺酰胺锂在交联剂存在的情况下进行乳液共聚而实现的,电子显微镜显示其粒径小于 30 纳米。然后,将不同磺酰胺锂含量的聚合物 NP 与不同的增塑剂(如碳酸盐和磺烷)混合,制备出聚合物电解质。聚合物电解质在 85 °C 时的离子电导率介于 2.9 × 10-4 和 2.3 × 10-5 S cm-1 之间,其中锂含量最高的小尺寸 NPs 的离子电导率值最高。作为概念验证应用,通过将油墨直接写入传统电池电极,对逐层打印砜基聚合物电解质进行了评估。印刷固体电解质的电化学特性表明,它具有良好的性能、离子电导率、锂转移数量、电化学稳定性窗口以及在锂对称电池中的可循环性,可用于锂电池。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


