Photoinduced radical cascade brominative addition/spirocyclization of N-arylpropiolamides and CBr4 with O2 as oxidant
引用次数: 0
Abstract
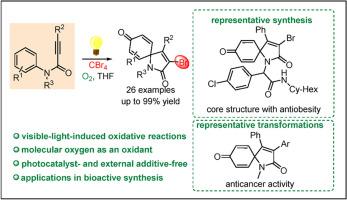
A visible-light-induced brominated spirocyclization of N-arylpropiolamides and CBr4 for the synthesis of 3-bromo-azaspiro[4,5]trienones is reported here. This process allows the formation of C–Br, C–C, and C=O bonds in a single reaction via a cascade radical addition/ipso-cyclization/oxidative dearomatization sequence. This protocol also features high functional group tolerance, operational simplicity and the use of molecular oxygen as an oxidant as well as sustainable photocatalyst- and additive-free reaction conditions at room temperature. Meanwhile, the presented straightforward and sustainable strategy has also been applied to the synthesis of several biologically active compounds.

以 O2 为氧化剂,光诱导 N-芳基丙炔酰胺和 CBr4 的自由基级联溴化加成/螺环化反应
本文报告了一种可见光诱导的 N-芳基丙炔酰胺和 CBr4 的溴化螺环化反应,用于合成 3-溴-氮杂螺[4,5]三烯酮。该工艺通过级联自由基加成/异环化/氧化脱芳烃顺序,在单个反应中形成 C-Br、C-C 和 C=O 键。该方案还具有官能团容限高、操作简单、使用分子氧作为氧化剂以及室温下可持续的无光催化剂和无添加剂反应条件等特点。同时,所介绍的这种简单、可持续的策略还被应用于多种生物活性化合物的合成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


