Scaling Up FluidFlower Results for Carbon Dioxide Storage in Geological Media
Abstract
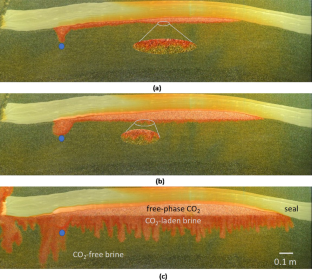
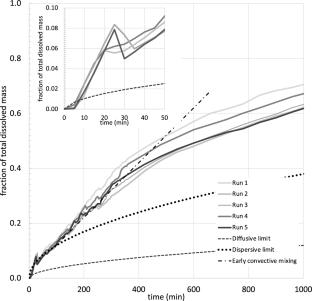
The partial differential equations describing immiscible, but soluble, carbon dioxide (CO2) displacement of brine in saline storage formations are developed including mass transfer across the CO2–brine interface. Scaling relationships for characteristic time among laboratory and representative storage formation conditions are found upon assumption that free-phase CO2 transport during injection is dominated by pressure-driven flow. The implication is that an hour in the FluidFlower (room-scale visual model) scales to hundreds of years of elapsed time in the storage formation. The scaling criteria permit extrapolation of the effects of changes in parameters and operating conditions. Interphase mass transfer allows CO2 to saturate the brine phase and the finite time of such mass transfer results in substantial time to approach equilibrium. Significant mixing of CO2 dissolved into formation brine with original brine is found experimentally and is also predicted. The magnitude of onset time for buoyancy-driven fingers that enhance mixing of CO2 is typically only a fraction of the duration of CO2 injection and in general agreement with theoretical analysis in the literature. Predictions for onset time of convective mixing at representative storage formation conditions, likewise, teach that the onset time for fingering is significantly less than the duration of CO2 injection in some cases. The implications of this observation include that mixing of CO2 with brine and the subsequent settling due to gravity are relatively rapid and coincide with the period of active CO2 injection.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


