Influence of the Morphology of the Surface of Titanium Dioxide Nanocrystallites on Their Catalytic Properties in the Alcohol Conversion Reactions
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
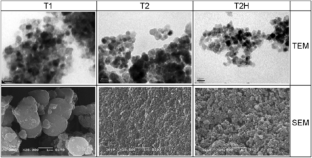
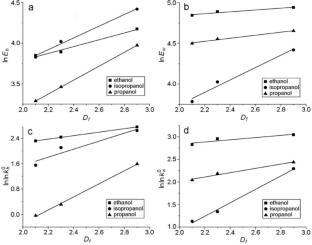
By using a fractal approach, it is found that the irregular structure of the surface of titanium dioxide-based catalyst has a significant effect on the values of the rate constants and activation energies of the reaction of dehydrogenation and dehydration of ethanol, n-propanol, and iso-propanol. Kinetics of ethyl and propyl alcohol conversions on titanium dioxide samples with different textures and morphologies is studied. Dependencies of activation energy and pre-exponential factor of the reaction rate on the alcohol conversions on the TiO2 samples on their structural characteristics, particularly a fractal dimension, are established.
二氧化钛纳米晶表面形态对其在酒精转化反应中催化特性的影响
通过使用分形方法,研究发现二氧化钛基催化剂表面的不规则结构对乙醇、正丙醇和异丙醇脱氢和脱水反应的速率常数和活化能值有显著影响。研究了不同质地和形态的二氧化钛样品上乙醇和丙醇的转化动力学。研究确定了二氧化钛样品上酒精转化率的活化能和前指数因数与其结构特征(特别是分形维度)的关系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Theoretical and Experimental Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.60
自引率
10.00%
发文量
30
审稿时长
6-12 weeks
期刊介绍:
Theoretical and Experimental Chemistry is a journal for the rapid publication of research communications and reviews on modern problems of physical chemistry such as:
a) physicochemical bases, principles, and methods for creation of novel processes, compounds, and materials;
b) physicochemical principles of chemical process control, influence of external physical forces on chemical reactions;
c) physical nanochemistry, nanostructures and nanomaterials, functional nanomaterials, size-dependent properties of materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


