Fe–Ni-based alloys as highly active and low-cost oxygen evolution reaction catalyst in alkaline media
IF 37.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
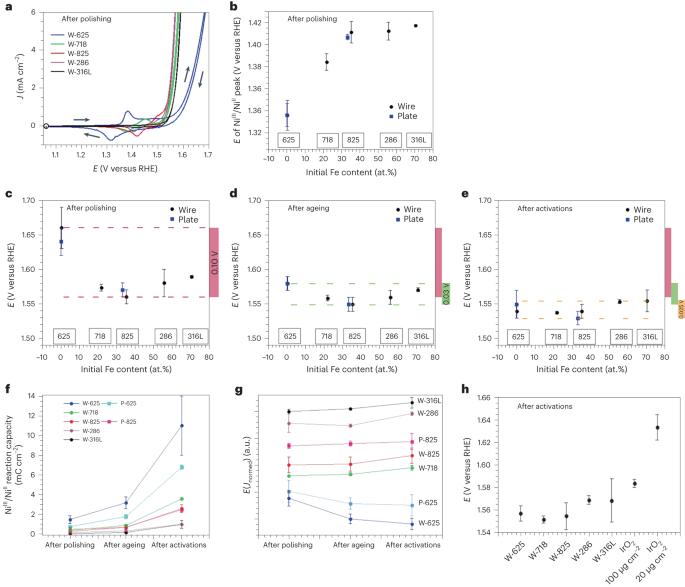
NiFe-based oxo-hydroxides are highly active for the oxygen evolution reaction but require complex synthesis and are poorly durable when deposited on foreign supports. Herein we demonstrate that easily processable, Earth-abundant and cheap Fe–Ni alloys spontaneously develop a highly active NiFe oxo-hydroxide surface, exsolved upon electrochemical activation. While the manufacturing process and the initial surface state of the alloys do not impact the oxygen evolution reaction performance, the growth/composition of the NiFe oxo-hydroxide surface layer depends on the alloying elements and initial atomic Fe/Ni ratio, hence driving oxygen evolution reaction activity. Whatever the initial Fe/Ni ratio of the Fe–Ni alloy (varying between 0.004 and 7.4), the best oxygen evolution reaction performance (beyond that of commercial IrO2) and durability was obtained for a surface Fe/Ni ratio between 0.2 and 0.4 and includes numerous active sites (high NiIII/NiII capacitive response) and high efficiency (high Fe/Ni ratio). This knowledge paves the way to active and durable Fe–Ni alloy oxygen-evolving electrodes for alkaline water electrolysers. NiFe-based oxo-hydroxides are active for the oxygen evolution reaction but suffer from complex synthesis and durability when deposited. Easily processable Fe–Ni alloys with a highly active oxo-hydroxide surface are now shown to pave the way for oxygen-evolving electrodes for alkaline water electrolysers.
作为碱性介质中高活性、低成本氧进化反应催化剂的铁镍基合金
镍铁基氧化氢氧化物在氧进化反应中具有很高的活性,但需要复杂的合成工艺,而且沉积在外来支撑物上时耐久性很差。在这里,我们证明了易于加工、富集于地球且廉价的铁镍合金可自发形成高活性的镍铁氧化氢氧化物表面,并在电化学活化后溶解。虽然合金的制造工艺和初始表面状态不会影响氧进化反应的性能,但镍铁氧化氢氧化物表层的生长/组成取决于合金元素和初始原子铁/镍比,从而推动氧进化反应的活性。无论铁-镍合金的初始铁/镍比(在 0.004 和 7.4 之间变化)如何,表面铁/镍比在 0.2 和 0.4 之间时,氧进化反应性能最好(超过商用二氧化铱),耐久性最好,包括大量活性位点(高 NiIII/NiII 电容响应)和高效率(高铁/镍比)。这些知识为碱性水电解槽的活性和耐用铁镍合金氧发生电极铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


