Multi-scale physics of bipolar membranes in electrochemical processes
引用次数: 0
Abstract
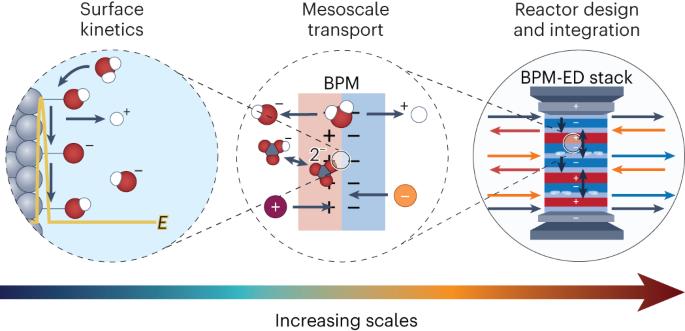
Bipolar membranes (BPMs) enable control of ion concentrations and fluxes in electrochemical cells suitable for a wide range of applications. Here we present the multi-scale physics of BPMs in an electrochemical engineering context and articulate design principles to drive the development of advanced BPMs. The chemistry, structure, and physics of BPMs are illustrated and related to the thermodynamics, transport phenomena, and chemical kinetics that dictate ion and species fluxes and selectivity. These interactions give rise to emergent structure–property–performance relationships that yield design criteria for BPMs that achieve high permselectivity, durability, and voltaic efficiency. The resulting performance trade-offs for BPMs are presented in the context of emerging applications in energy conversion or storage, and environmental remediation. By connecting the fundamental physical phenomena in BPMs to device-level performance and engineering, we aim to facilitate the development of next-generation BPMs for sustainable electrochemical processes. Bipolar ion-exchange membranes are a class of charged polymers that enable precise control of ionic fluxes and local pH, making them potentially valuable for many energy and environmental applications. This Review focuses on the fundamental physics underpinning their operation across multiple scales, from nanomorphology to integration within devices such as in bipolar-membrane electrodialysis (BPM-ED).
电化学过程中的双极膜多尺度物理学
双极膜(BPM)能够控制电化学电池中的离子浓度和流量,适用于广泛的应用领域。在此,我们从电化学工程的角度介绍了双极膜的多尺度物理学原理,并阐明了推动先进双极膜发展的设计原则。我们阐述了 BPM 的化学、结构和物理学,并将其与决定离子和物种通量及选择性的热力学、传输现象和化学动力学联系起来。这些相互作用产生了新的结构-属性-性能关系,从而产生了实现高选择性、耐用性和伏特效率的 BPM 设计标准。由此产生的 BPM 性能权衡,将在能源转换或存储以及环境修复等新兴应用的背景下进行介绍。通过将 BPM 的基本物理现象与设备级性能和工程学联系起来,我们旨在促进下一代 BPM 的开发,以实现可持续的电化学过程。双极离子交换膜是一类带电聚合物,能够精确控制离子通量和局部 pH 值,因此对许多能源和环境应用具有潜在价值。本综述重点介绍了从纳米形态学到集成到双极膜电渗析(BPM-ED)等设备中的多尺度操作基础物理学。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


