Carbon–carbon bond cleavage for a lignin refinery
引用次数: 0
Abstract
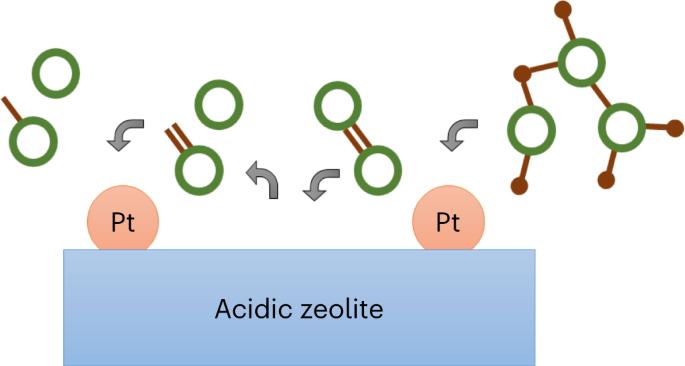
Carbon–carbon bonds, ubiquitous in lignin, limit monomer yields from current depolymerization strategies, which mainly target C–O bonds. Selective cleavage of the inherently inert σ-type C–C bonds without pre-functionalization remains challenging. Here we report the breaking of C–C bonds in lignin obtained upon initial disruption of labile C–O bonds, achieving monocyclic hydrocarbon yields up to an order of magnitude higher than previously reported. The use of a Pt (de)hydrogenation function leads to olefinic groups close to recalcitrant C–C bonds, which can undergo β-scission over zeolitic Brønsted acid sites. After confirming that this approach can selectively cleave common C–C linkages (5–5′, β–1′, β–5′ and β–β′) in lignin skeletons, we demonstrate its utility in the valorization of various representative lignins. A techno-economic analysis shows the promise of our method for producing gasoline- and jet-range cycloalkanes and aromatics, while a life-cycle assessment confirms its potential for CO2-neutral fuel production. Carbon–carbon bonds are ubiquitous in lignin, limiting monomer yields from current depolymerization strategies mainly targeting C–O bonds. Now, a bifunctional hydrocracking approach uses a Pt/zeolite catalyst to break C–C bonds in lignin waste, achieving monocyclic hydrocarbon yields up to 54 C%.
木质素精炼厂的碳-碳键裂解技术
木质素中无处不在的碳-碳键限制了目前主要针对 C-O 键的解聚策略的单体产量。在不进行预官能化的情况下选择性地裂解固有惰性的 σ 型 C-C 键仍然具有挑战性。在此,我们报告了木质素中的 C-C 键在最初破坏易变的 C-O 键时被断裂的情况,其单环烃产量比之前报道的高出一个数量级。铂(脱)氢化功能的使用导致烯烃基团靠近难处理的 C-C 键,这些烯烃基团可以在沸石布氏酸位点上发生 β 裂解。在证实这种方法可以选择性地裂解木质素骨架中常见的 C-C 键(5-5′、β-1′、β-5′ 和 β-β′)后,我们展示了这种方法在各种代表性木质素价值化中的实用性。技术经济分析表明,我们的方法有望生产汽油和喷气燃料范围内的环烷烃和芳烃,而生命周期评估则证实了其在二氧化碳中和燃料生产方面的潜力。碳-碳键在木质素中无处不在,限制了目前主要针对 C-O 键的解聚策略的单体产量。现在,一种双功能加氢裂化方法使用铂/沸石催化剂来断裂木质素废料中的 C-C 键,使单环烃产量高达 54 C%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


