ICSH review of internal quality control policy for blood cell counters
Abstract
Introduction
This paper is a report of an ICSH review of policies and practices for internal quality control (IQC) policy for haematology cell counters among regulatory bodies, cell counter manufacturers and diagnostic laboratories. It includes a discussion of the study findings and links to separate ICSH guidance for such policies and practices. The application of internal quality control (IQC) methods is an essential pre-requisite for all clinical laboratory testing including the blood count (Full Blood Count, FBC, or Complete Blood Count, CBC).
Methods
The ICSH has gathered information regarding the current state of practice through review of published guidance from regulatory bodies, a questionnaire to six major cell counter manufacturers (Abbott Diagnostics, Beckman Coulter, Horiba Medical Diagnostic Instruments & Systems, Mindray Medical International, Siemens Healthcare Diagnostics and Sysmex Corporation) and a survey issued to 191 diagnostic laboratories in four countries (China, Republic of Ireland, Spain and the United Kingdom) on their IQC practice and approach to use of commercial IQC materials.
Results
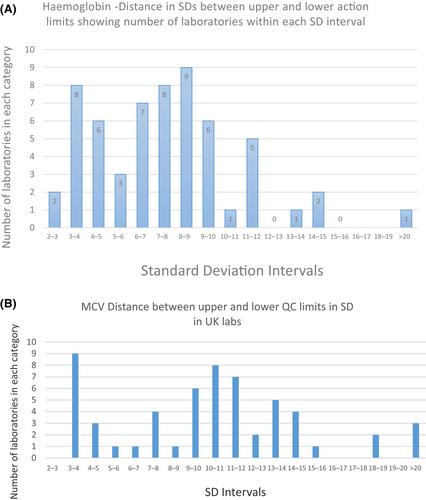
This has revealed diversity both in guidance and in practice around the world. There is diversity in guidance from regulatory organizations in regard to IQC methods each recommends, clinical levels to use and frequency to run commercial controls, and finally recommended sources of commercial controls. The diversity in practice among clinical laboratories spans the areas of IQC methods used, derivation of target values and action limits used with control materials, and frequency of running commercial controls materials.
Conclusions
These findings and their implications for IQC Practice are discussed in this paper. They are used to inform a separate guidance document, which proposes a harmonized approach to address the issues faced by diagnostic laboratories.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


