Ruthenium-catalysed direct C–H amidation of 4-aryl-pyrrolo[2,3-d]pyrimidines with acyl/phosphoryl azides†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A ruthenium-catalysed arene ortho C–H amidation of 4-aryl-pyrrolo[2,3-d]pyrimidine derivatives with acyl azides or phosphoryl azides as the nitrogen sources toward C–N bond formation was developed. This protocol could offer a novel and direct approach to access a series of amidated and phosphoramidated pyrrolo[2,3-d]pyrimidine derivatives in moderate to good yields, thereby evading the general Curtius rearrangement. The protocol features significant functional group tolerance and a single-step process, with the release of only innocuous molecular nitrogen as the byproduct.

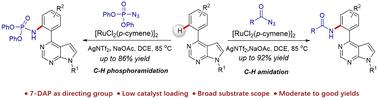
钌催化的 4-芳基吡咯并[2,3-d]嘧啶与酰基/磷酸叠氮化物的直接 C-H 酰胺化反应。
以酰基叠氮化物或磷酰叠氮化物为氮源,开发了一种钌催化的 4-芳基吡咯并[2,3-d]嘧啶衍生物的正交 C-H 酰胺化反应,以形成 C-N 键。该方案提供了一种新颖而直接的方法,可以中等至高产率获得一系列酰胺化和磷酰胺化的吡咯并[2,3-d]嘧啶衍生物,从而避免了一般的柯蒂乌斯重排。该方案具有明显的官能团耐受性和单步过程,只释放出无害的分子氮作为副产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


