Three-dimensional printed biomimetic multilayer scaffolds coordinated with sleep-related small extracellular vesicles: A strategy for extracellular matrix homeostasis and macrophage polarization to enhance osteochondral regeneration
IF 8.5
4区 医学
Q1 MATERIALS SCIENCE, BIOMATERIALS
引用次数: 0
Abstract
Cartilage defects resulting from injury or degeneration are a common clinical problem, and due to its avascular nature, articular cartilage has poor self-healing capacity. Three-dimensional (3D) bioprinting has attracted great attention in tissue engineering. Melatonin (MT), a hormone mainly secreted at night, plays an important role in tissue repair. Small extracellular vesicles (sEV) are considered ideal drug delivery vehicles and MT-sEV (sleep-related sEV) have the potential ability to promote cartilage regeneration. Here, biomimetic multilayer scaffolds were fabricated using 3D bioprinting. A double network hydrogel, composed of methacrylated hyaluronic acid and gelatin methacryloyl (HG), was prepared. MT-sEV and HG hydrogel were used to create a cartilage layer. A bone layer was formed using poly(ε-caprolactone) and hydroxyapatite ultralong nanowires. Additionally, two bioinks were alternately printed at the interface layer. The results of RNA sequencing revealed the potential regulatory mechanisms. MT-sEV showed promotional effects on cell migration, proliferation, chondrogenic differentiation, and extracellular matrix (ECM) deposition. Moreover, MT-sEV altered macrophage polarization and regulated the expression of inflammatory cytokines. In vivo experiments demonstrated that the biomimetic multilayer scaffolds promoted cartilage regeneration. These experiments demonstrated the ability of MT-sEV to regulate the immune microenvironment and promote the secretion of ECM, providing a promising strategy for cartilage regeneration.
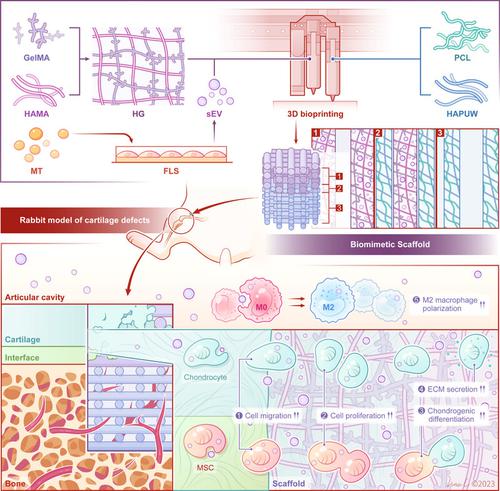
与睡眠相关的细胞外基质小泡协调的三维打印仿生多层支架:促进细胞外基质平衡和巨噬细胞极化以增强骨软骨再生的策略
软骨损伤或退行性变导致的软骨缺损是一种常见的临床问题,由于其无血管性质,关节软骨的自我愈合能力较差。三维(3D)生物打印技术在组织工程领域备受关注。褪黑素(MT)是一种主要在夜间分泌的激素,在组织修复中发挥着重要作用。细胞外小泡(sEV)被认为是理想的药物输送载体,而MT-sEV(与睡眠相关的sEV)具有促进软骨再生的潜在能力。在这里,我们利用三维生物打印技术制作了仿生物多层支架。制备的双层网络水凝胶由甲基丙烯酸化透明质酸和甲基丙烯酰明胶(HG)组成。MT-sEV 和 HG 水凝胶用于创建软骨层。使用聚(ε-己内酯)和羟基磷灰石超长纳米线形成骨层。此外,还在界面层交替印制了两种生物墨水。RNA 测序结果揭示了潜在的调控机制。MT-sEV 对细胞迁移、增殖、软骨分化和细胞外基质(ECM)沉积有促进作用。此外,MT-sEV 还能改变巨噬细胞的极化,调节炎症细胞因子的表达。体内实验表明,仿生多层支架促进了软骨再生。这些实验证明了 MT-sEV 调节免疫微环境和促进 ECM 分泌的能力,为软骨再生提供了一种前景广阔的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

VIEW
Multiple-
CiteScore
12.60
自引率
2.30%
发文量
0
审稿时长
10 weeks
期刊介绍:
View publishes scientific articles studying novel crucial contributions in the areas of Biomaterials and General Chemistry. View features original academic papers which go through peer review by experts in the given subject area.View encourages submissions from the research community where the priority will be on the originality and the practical impact of the reported research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


