Inhalable extracellular vesicle delivery of IL-12 mRNA to treat lung cancer and promote systemic immunity
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Lung carcinoma is one of the most common cancers and has one of the lowest survival rates in the world. Cytokines such as interleukin-12 (IL-12) have demonstrated considerable potential as robust tumour suppressors. However, their applications are limited due to off-target toxicity. Here we report on a strategy involving the inhalation of IL-12 messenger RNA, encapsulated within extracellular vesicles. Inhalation and preferential uptake by cancer cells results in targeted delivery and fewer systemic side effects. The IL-12 messenger RNA generates interferon-γ production in both innate and adaptive immune-cell populations. This activation consequently incites an intense activation state in the tumour microenvironment and augments its immunogenicity. The increased immune response results in the expansion of tumour cytotoxic immune effector cells, the formation of immune memory, improved antigen presentation and tumour-specific T cell priming. The strategy is demonstrated against primary neoplastic lesions and provides profound protection against subsequent tumour rechallenge. This shows the potential for locally delivered cytokine-based immunotherapies to address orthotopic and metastatic lung tumours. Cytokine interleukin-12 (IL-12) has potential for tumour suppression yet off-target effects limit potential applications. Here the authors report on the delivery of IL-12 mRNA encapsulated in extracellular vesicles to lungs via inhalation and demonstrate the immunotherapeutic potential of targeted cytokine mRNA therapy.
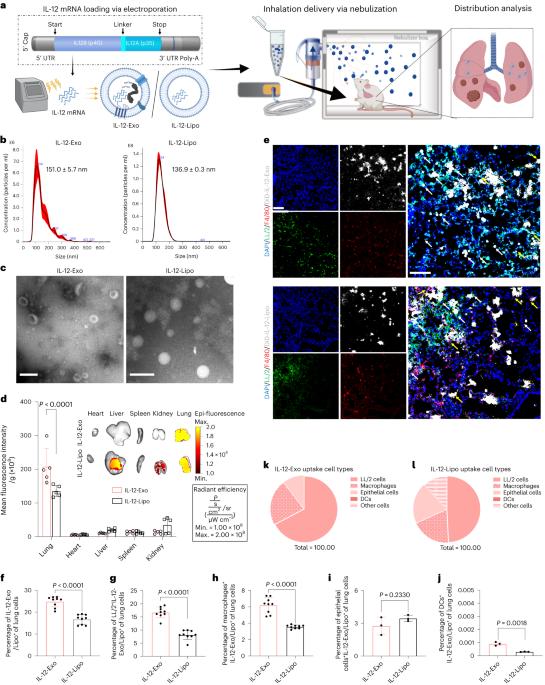
通过吸入细胞外囊泡输送 IL-12 mRNA 治疗肺癌并促进全身免疫力
肺癌是最常见的癌症之一,也是世界上存活率最低的癌症之一。白细胞介素-12(IL-12)等细胞因子作为强大的肿瘤抑制剂已显示出相当大的潜力。然而,由于脱靶毒性,它们的应用受到了限制。在此,我们报告了一种吸入包裹在细胞外囊泡中的 IL-12 信使 RNA 的策略。通过吸入并被癌细胞优先吸收,可实现靶向给药并减少全身副作用。IL-12 信使 RNA 可在先天性和适应性免疫细胞群中产生干扰素-γ。这种激活会在肿瘤微环境中引发强烈的活化状态,并增强其免疫原性。免疫反应的增强会导致肿瘤细胞毒性免疫效应细胞的扩增、免疫记忆的形成、抗原递呈的改善以及肿瘤特异性 T 细胞的启动。该策略针对原发性肿瘤病变进行了论证,并对随后的肿瘤再侵袭提供了深远的保护。这表明,基于细胞因子的局部给药免疫疗法具有治疗原位和转移性肺肿瘤的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


