Activation of neutrophils excels the therapeutic potential of Mycobacterium indicus pranii and heat-induced promastigotes against antimony-resistant Leishmania donovani infection
IF 1.6
4区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
Repurposing drugs and adjuvants is an attractive choice of present therapy that reduces the substantial costs, chances of failure, and systemic toxicity. Mycobacterium indicus pranii was originally developed as a leprosy vaccine but later has been found effective against Leishmania donovani infection. To extend our earlier study, here we reported the immunotherapeutic modulation of the splenic and circulatory neutrophils in favour of hosts as neutrophils actually serve as the pro-parasitic portable shelter to extend the Leishmania infection specifically during the early entry into the hosts' circulation. We targeted to disrupt this early pro-parasitic incidence by the therapeutic combination of M. indicus pranii and heat-induced promastigotes against antimony-resistant L. donovani infection. The combination therapy induced the functional expansion of CD11b+Ly6CintLy6Ghi neutrophils both in the post-infected spleen, and also in the circulation of post-treated animals followed by the immediate Leishmania infection. More importantly, the enhanced expression of MHC-II, phagocytic uptake of the parasites by the circulatory neutrophils as well as the oxidative burst were induced that limited the chances of the very early establishment of the infection. The enhanced expression of pro-inflammatory cytokines, like IL-1α and TNF-α indicated resistance to the parasite-mediated takeover of the neutrophils, as these cytokines are critical for the activation of T cell-mediated immunity and host-protective responses. Additionally, the induction of essential transcription factors and cytokines for early granulocytic lineage commitment suggests that the strategy not only contributed to the peripheral activation of the neutrophils but also promoted granulopoiesis in the bone marrow.
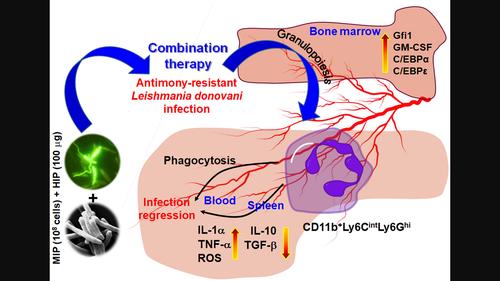
激活中性粒细胞可增强胰分枝杆菌和热诱导原核细胞对抗生素耐药的唐氏利什曼病感染的治疗潜力
药物和佐剂的再利用是目前治疗的一种有吸引力的选择,它可以降低大量成本、失败几率和全身毒性。普拉尼分枝杆菌最初是作为麻风病疫苗开发的,但后来发现它对唐氏利什曼原虫感染有效。为了扩展我们之前的研究,我们在此报告了对脾脏和循环中性粒细胞的免疫治疗调节,以利于宿主,因为中性粒细胞实际上是亲寄生虫的便携式庇护所,特别是在早期进入宿主循环的过程中扩大利什曼病感染。我们的目标是通过将嗜中性粒细胞和热诱导原核细胞结合起来治疗抗锑抗性利什曼病感染,来破坏这种早期原寄生虫的发生。联合疗法诱导 CD11b+Ly6CintLy6Ghi 中性粒细胞在感染后的脾脏中以及在利什曼原虫感染后的动物血液循环中功能性扩增。更重要的是,MHC-II 表达的增强、循环中性粒细胞对寄生虫的吞噬摄取以及氧化猝灭的诱导限制了早期感染的机会。IL-1α和TNF-α等促炎细胞因子的表达增强,表明中性粒细胞对寄生虫介导的接管具有抵抗力,因为这些细胞因子对激活T细胞介导的免疫和宿主保护反应至关重要。此外,诱导早期粒细胞系形成所必需的转录因子和细胞因子表明,该策略不仅有助于中性粒细胞的外周活化,还能促进骨髓中的粒细胞生成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
5.40%
发文量
109
审稿时长
1 months
期刊介绍:
This peer-reviewed international journal publishes original articles and reviews on all aspects of basic, translational and clinical immunology. The journal aims to provide high quality service to authors, and high quality articles for readers.
The journal accepts for publication material from investigators all over the world, which makes a significant contribution to basic, translational and clinical immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


