Expanding the catalytic landscape of metalloenzymes with lytic polysaccharide monooxygenases
IF 38.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
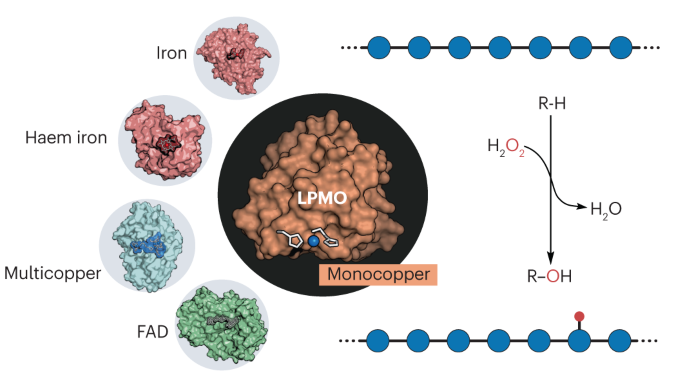
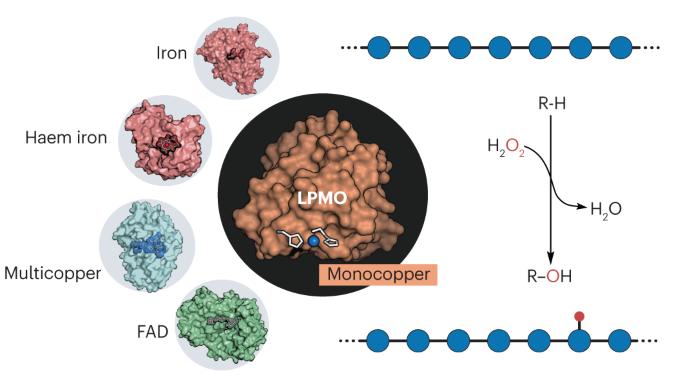
Lytic polysaccharide monooxygenases (LPMOs) have an essential role in global carbon cycle, industrial biomass processing and microbial pathogenicity by catalysing the oxidative cleavage of recalcitrant polysaccharides. Despite initially being considered monooxygenases, experimental and theoretical studies show that LPMOs are essentially peroxygenases, using a single copper ion and H2O2 for C–H bond oxygenation. Here, we examine LPMO catalysis, emphasizing key studies that have shaped our comprehension of their function, and address side and competing reactions that have partially obscured our understanding. Then, we compare this novel copper–peroxygenase reaction with reactions catalysed by haem iron enzymes, highlighting the different chemistries at play. We conclude by addressing some open questions surrounding LPMO catalysis, including the importance of peroxygenase and monooxygenase reactions in biological contexts, how LPMOs modulate copper site reactivity and potential protective mechanisms against oxidative damage. Lytic polysaccharide monooxygenases are key enzymes in biomass processing and pathogenicity. They are, to our knowledge, the first known copper enzymes capable of utilizing H2O2 to hydroxylate C–H bonds. This Review draws a portrait of the catalytic paths at play and highlights outstanding questions in their reactivity.
用溶解多糖单加氧酶扩展金属酶的催化范围
溶解性多糖单加氧酶(LPMOs)通过催化难溶性多糖的氧化裂解,在全球碳循环、工业生物质加工和微生物致病性方面发挥着重要作用。尽管 LPMO 最初被认为是单加氧酶,但实验和理论研究表明,LPMO 本质上是过氧酶,使用单个铜离子和 H2O2 进行 C-H 键加氧。在此,我们将探讨 LPMO 催化作用,强调影响我们对其功能理解的关键研究,并讨论部分模糊了我们的理解的副反应和竞争反应。然后,我们将这种新颖的铜过氧酶反应与血红素铁酶催化的反应进行比较,突出强调其中不同的化学作用。最后,我们探讨了围绕 LPMO 催化作用的一些悬而未决的问题,包括过氧酶和单氧酶反应在生物环境中的重要性、LPMO 如何调节铜位点反应性以及防止氧化损伤的潜在保护机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


