Tumour-infiltrating lymphocyte therapy for patients with advanced-stage melanoma
IF 81.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Immunotherapy with immune-checkpoint inhibitors (ICIs) and targeted therapy with BRAF and MEK inhibitors have revolutionized the treatment of melanoma over the past decade. Despite these breakthroughs, the 5-year survival rate of patients with advanced-stage melanoma is at most 50%, emphasizing the need for additional therapeutic strategies. Adoptive cell therapy with tumour-infiltrating lymphocytes (TILs) is a therapeutic modality that has, in the past few years, demonstrated long-term clinical benefit in phase II/III trials involving patients with advanced-stage melanoma, including those with disease progression on ICIs and/or BRAF/MEK inhibitors. In this Review, we summarize the current status of TIL therapies for patients with advanced-stage melanoma, including potential upcoming marketing authorization, the characteristics of TIL therapy products, as well as future strategies that are expected to increase the efficacy of this promising cellular immunotherapy. Despite dramatic progress over the past decade, only around 50% of patients with advanced-stage melanoma derive durable benefit from immune-checkpoint inhibitors (ICIs) and/or BRAF and MEK (BRAF/MEK) inhibitors. Over the past few years, adoptive cell therapy with tumour-infiltrating lymphocytes (TILs) has demonstrated encouraging efficacy including in patients with disease progression on ICIs or BRAF/MEK inhibitors. In this Review, the authors summarize the role of TIL therapies in the management of these patients and describe future research strategies that might improve safety or efficacy.
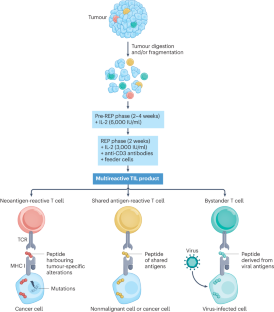
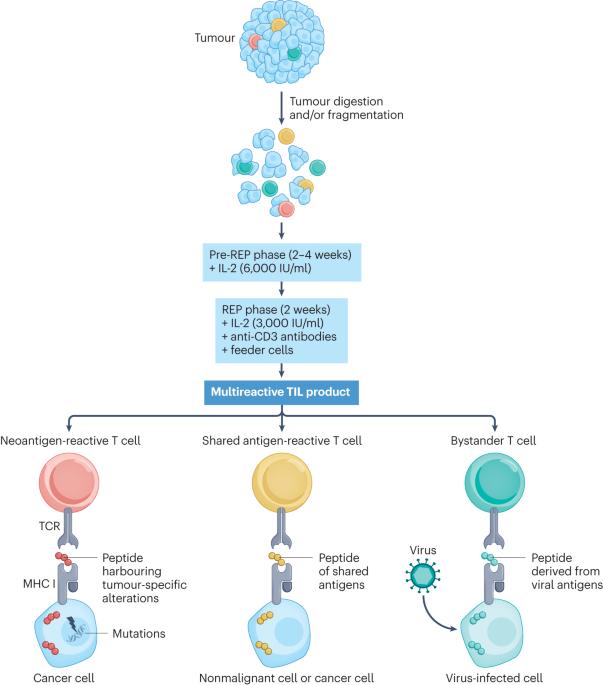
晚期黑色素瘤患者的肿瘤浸润淋巴细胞疗法
过去十年来,免疫检查点抑制剂(ICIs)的免疫疗法以及BRAF和MEK抑制剂的靶向疗法彻底改变了黑色素瘤的治疗方法。尽管取得了这些突破性进展,但晚期黑色素瘤患者的 5 年存活率最多只有 50%,因此需要更多的治疗策略。使用肿瘤浸润淋巴细胞(TILs)进行的适应性细胞疗法是一种治疗方式,过去几年中,这种疗法在涉及晚期黑色素瘤患者(包括使用 ICIs 和/或 BRAF/MEK 抑制剂后病情进展的患者)的 II/III 期试验中显示出了长期临床疗效。在本综述中,我们将总结治疗晚期黑色素瘤患者的 TIL 疗法的现状,包括可能即将获得的上市许可、TIL 疗法产品的特点以及有望提高这种前景广阔的细胞免疫疗法疗效的未来策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


