HspBP1 in Complex with the Peptide of the Innate Immunity Protein Tag7 is Able to Lyse Tumor Cells Carrying TNFR1 Receptor
IF 0.8
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The search for new cytotoxic agents capable of lysing tumor cells is an important task in the fight against cancer. Here we have shown that the HspBP1 protein, the chaperone of the heat shock protein Hsp70, is able to form a complex with the previously discovered peptide (17.1) of the innate immunity protein Tag7. Experiments using thermophoresis demonstrated that the affinity of the Tag7 protein peptide 17.1 to the HspBP1 molecule is 100 times higher than that of the full-sized Tag7 molecule. The addition of the 17.1–HspBP1 complex to tumor cells induces apoptosis and necroptosis in them. The results obtained in this work can be used to develop promising antitumor drugs.
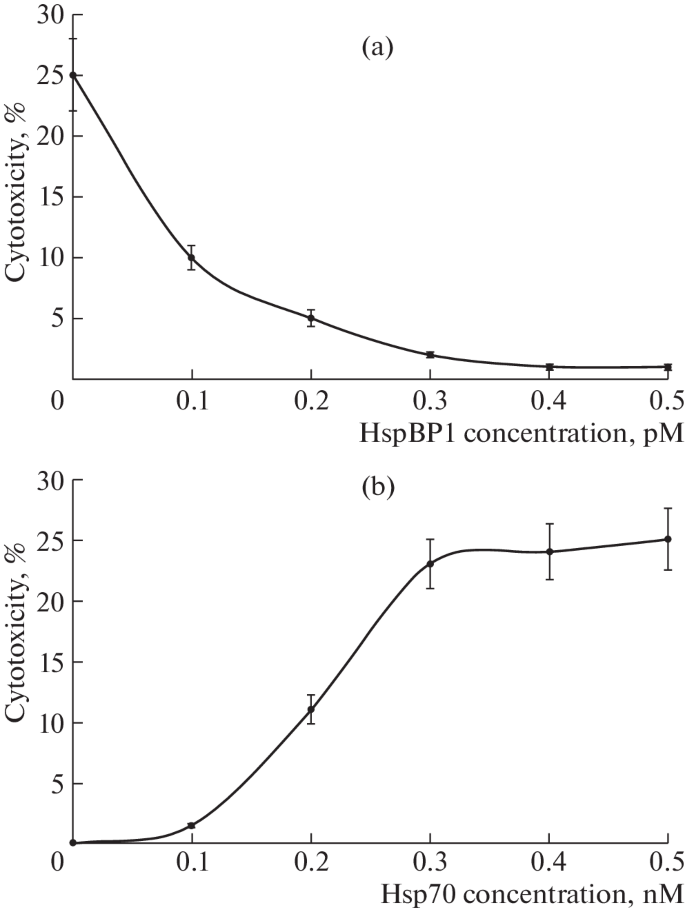
HspBP1 与先天性免疫蛋白 Tag7 的多肽复合物能溶解携带 TNFR1 受体的肿瘤细胞。
寻找能够溶解肿瘤细胞的新型细胞毒剂是抗击癌症的一项重要任务。在这里,我们发现热休克蛋白 Hsp70 的伴侣蛋白 HspBP1 能够与之前发现的先天免疫蛋白 Tag7 的多肽(17.1)形成复合物。利用热电泳技术进行的实验表明,Tag7 蛋白多肽 17.1 与 HspBP1 分子的亲和力比完整尺寸的 Tag7 分子高 100 倍。在肿瘤细胞中加入 17.1-HspBP1 复合物可诱导细胞凋亡和坏死。这项研究的结果可用于开发有前景的抗肿瘤药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Doklady Biochemistry and Biophysics
生物-生化与分子生物学
CiteScore
1.60
自引率
12.50%
发文量
68
审稿时长
6-12 weeks
期刊介绍:
Doklady Biochemistry and Biophysics is a journal consisting of English translations of articles published in Russian in biochemistry and biophysics sections of the Russian-language journal Doklady Akademii Nauk. The journal''s goal is to publish the most significant new research in biochemistry and biophysics carried out in Russia today or in collaboration with Russian authors. The journal accepts only articles in the Russian language that are submitted or recommended by acting Russian or foreign members of the Russian Academy of Sciences. The journal does not accept direct submissions in English.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


