Boosting characteristic emissions of lanthanide in Cs2Ag0.6Na0.4InCl6–xBrx double perovskites via mixing halide
IF 7.2
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Lanthanide ions (Ln3+) doping provides a potential strategy to control over the luminescent properties of lead-free halide double perovskite nanocrystals (DP NCs). However, due to the low energy transfer efficiency between self-trapped exciton (STE) and Ln3+ ions, the characteristic emissions of Ln3+ ions are not prominent. Furthermore, the energy transfer mechanism between STE and Ln3+ ions is also elusive and requires in-depth study. We chose trace Bi3+-doped Cs2Ag0.6Na0.4InCl6–xBrx as a representative DP matrix to demonstrate that by tuning the bromide concentration, the Ln3+ emission can be greatly enhanced. Such enhanced STE and Ln3+ ions energy transfer originates from the high covalency of Ln–Br bond, which contributes to improvement of the characteristic emission of Ln3+ ions. Furthermore, optical spectroscopy reveals that the energy transfer mechanism from DP to Eu3+ ions is different from all the other doped Ln3+ ions. The energy transfer from DP to Eu3+ ions is mostly through Eu–Br charge transfer while the other Ln3+ ions are excited by energy transfer from STE. The distinct energy transfer mechanism has resulted from the energy separation between the excited energy level of Ln3+ ions and the bottom of conduction band of DP. With increasing the energy separation, the energy transfer from STE to Ln3+ ions is less efficient because of the generation of a larger number of phonons and finally becomes impossible for Eu3+ ions. Our results provide new insight into tuning the energy transfer of Ln3+-doped DP NCs.
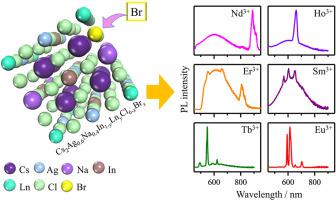
通过混合提高 Cs2Ag0.6Na0.4InCl6-xBrx 双包晶中镧系元素的特征发射率
掺杂镧系离子(Ln3+)为控制无铅卤化物双过氧化物纳米晶体(DP NCs)的发光特性提供了一种潜在的策略。然而,由于自俘获激子(STE)和 Ln3+ 离子之间的能量传递效率较低,Ln3+ 离子的发射特性并不突出。此外,STE 与 Ln3+ 离子之间的能量传递机制也难以捉摸,需要深入研究。我们选择了痕量 Bi3+ 掺杂的 Cs2Ag0.6Na0.4InCl6-xBrx 作为具有代表性的 DP 基质,以证明通过调整溴化物浓度,可以大大增强 Ln3+ 发射。这种 STE 和 Ln3+ 离子能量传递的增强源于 Ln-Br 键的高共价性,它有助于改善 Ln3+ 离子的发射特性。此外,光学光谱显示,从 DP 到 Eu3+ 离子的能量转移机制不同于所有其他掺杂的 Ln3+ 离子。从 DP 到 Eu3+ 离子的能量转移主要是通过 Eu-Br 电荷转移实现的,而其他 Ln3+ 离子则是通过 STE 的能量转移激发的。Ln3+ 离子的激发能级与 DP 的导带底部之间的能量分离导致了截然不同的能量转移机制。随着能量分离的增加,从 STE 到 Ln3+ 离子的能量转移效率会降低,因为会产生更多的声子,最终导致 Eu3+ 离子无法被激发。我们的研究结果为调整掺 Ln3+ 的 DP NC 的能量转移提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Rare Earths
化学-应用化学
CiteScore
8.70
自引率
14.30%
发文量
374
审稿时长
1.7 months
期刊介绍:
The Journal of Rare Earths reports studies on the 17 rare earth elements. It is a unique English-language learned journal that publishes works on various aspects of basic theory and applied science in the field of rare earths (RE). The journal accepts original high-quality original research papers and review articles with inventive content, and complete experimental data. It represents high academic standards and new progress in the RE field. Due to the advantage of abundant RE resources of China, the research on RE develops very actively, and papers on the latest progress in this field emerge every year. It is not only an important resource in which technicians publish and obtain their latest research results on RE, but also an important way of reflecting the updated progress in RE research field.
The Journal of Rare Earths covers all research and application of RE rare earths including spectroscopy, luminescence and phosphors, rare earth catalysis, magnetism and magnetic materials, advanced rare earth materials, RE chemistry & hydrometallurgy, RE metallography & pyrometallurgy, RE new materials, RE solid state physics & solid state chemistry, rare earth applications, RE analysis & test, RE geology & ore dressing, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


