B(C6F5)3-based Lewis pair-catalyzed acrylate polymerization: Lewis base effects on pairing interactions
IF 2.3
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
Abstract
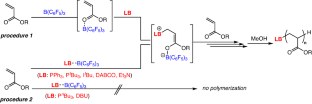
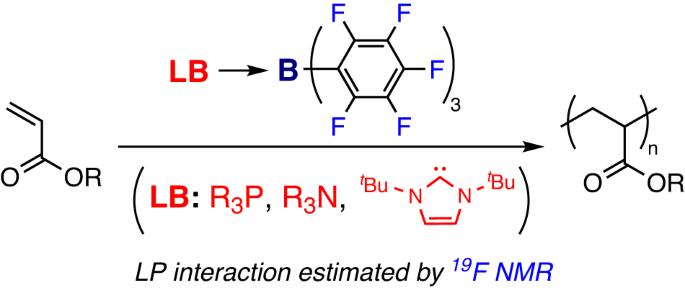
Acrylate polymerizations catalyzed by Lewis pairs (LP) composed of B(C6F5)3 and various Lewis bases (phosphines, amines, and an N-heterocyclic carbene) in dichloromethane were investigated using two procedures based on different monomer/catalyst addition sequences. In procedure 1, Lewis bases were added to B(C6F5)3-activated n-butyl acrylate (nBA), and the polymerization proceeded quantitatively using all Lewis bases at a wide temperature range (−60 °C to 30 °C). A low nucleophilic Lewis base Et3N also initiated the polymerization even at −60 °C. However, t-butyl acrylate was not polymerized, as LP promoted its conversion into acrylic acid and isobutene. In procedure 2, nBA was added to interacting LPs; the type of Lewis base significantly affected the polymerization results. Specifically, polymerization was not observed when 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and PnBu3 were applied; however, similar to the reaction in procedure 1, PPh3, PtBu3, and 1,4-diazabicyclo[2.2.2]octane (DABCO) initiated nBA polymerization. The pairing interactions between LBs/B(C6F5)3 (PPh3, Et3N, DBU, and DABCO) were investigated using the shift of 19F nuclear magnetic resonance signals, demonstrating that weak interacting LPs efficiently initiated the polymerizations in procedure 2. Acrylate polymerizations catalyzed by Lewis pairs (LP) composed of B(C6F5)3 and various Lewis bases were investigated using two procedures based on different monomer/catalyst addition sequences. When Lewis bases were added to B(C6F5)3-activated n-butyl acrylate (nBA) (procedure 1), the polymerization proceeded quantitatively using all Lewis bases. In contrast, the type of Lewis base significantly affected the polymerization results when nBA was added to interacting LPs (procedure 2). 19F nuclear magnetic resonance analysis of the LPs indicated that weakly interacting LPs efficiently initiated the polymerizations in procedure 2.
基于 B(C6F5)3 的路易斯配对催化丙烯酸酯聚合:路易斯碱对配对相互作用的影响
研究人员根据不同的单体/催化剂添加顺序,采用两种程序研究了由 B(C6F5)3 和各种路易斯碱(膦、胺和 N-杂环碳烯)组成的路易斯对(LP)在二氯甲烷中催化的丙烯酸酯聚合反应。在程序 1 中,路易斯碱被添加到 B(C6F5)3 活化的丙烯酸正丁酯(nBA)中,在较宽的温度范围(-60 °C 至 30 °C)内使用所有路易斯碱进行定量聚合。低亲核路易斯碱 Et3N 甚至在 -60 °C 时也能引发聚合反应。但是,丙烯酸叔丁酯没有聚合,因为 LP 会促进其转化为丙烯酸和异丁烯。在步骤 2 中,nBA 被添加到相互作用的 LP 中;路易斯碱的类型对聚合结果有显著影响。具体来说,当使用 1,8-二氮杂双环[5.4.0]十一-7-烯(DBU)和 PnBu3 时,没有观察到聚合反应;然而,与步骤 1 中的反应类似,PPh3、PtBu3 和 1,4-二氮杂双环[2.2.2]辛烷(DABCO)可引发 nBA 聚合反应。利用 19F 核磁共振信号的移动研究了 LBs/B(C6F5)3(PPh3、Et3N、DBU 和 DABCO)之间的配对相互作用,结果表明弱相互作用的 LPs 能有效地引发程序 2 中的聚合反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


