Candida albicans extracellular vesicles trigger type I IFN signalling via cGAS and STING
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
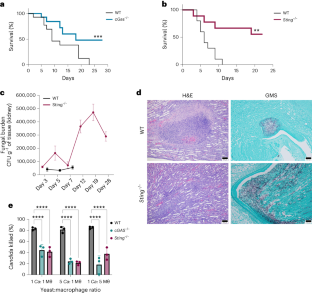
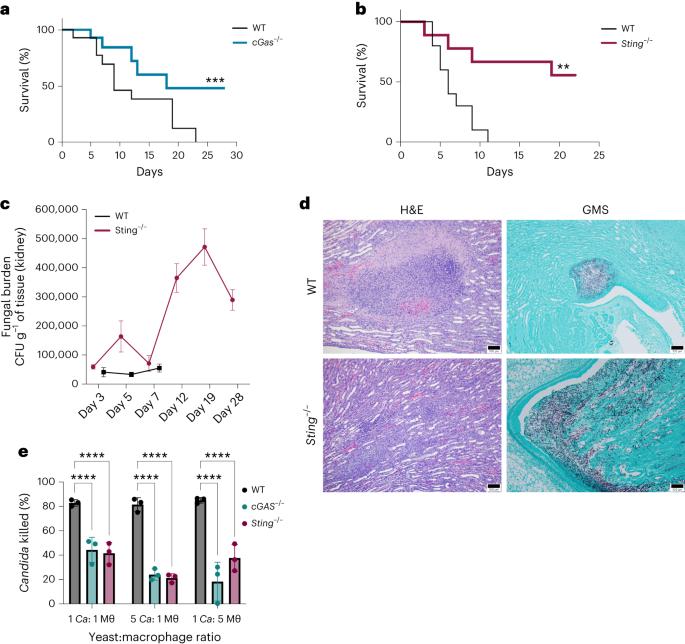
The host type I interferon (IFN) pathway is a major signature of inflammation induced by the human fungal pathogen, Candida albicans. However, the molecular mechanism for activating this pathway in the host defence against C. albicans remains unknown. Here we reveal that mice lacking cyclic GMP–AMP synthase (cGAS)–stimulator of IFN genes (STING) pathway components had improved survival following an intravenous challenge by C. albicans. Biofilm-associated C. albicans DNA packaged in extracellular vesicles triggers the cGAS–STING pathway as determined by induction of interferon-stimulated genes, IFNβ production, and phosphorylation of IFN regulatory factor 3 and TANK-binding kinase 1. Extracellular vesicle-induced activation of type I IFNs was independent of the Dectin-1/Card9 pathway and did not require toll-like receptor 9. Single nucleotide polymorphisms in cGAS and STING potently altered inflammatory cytokine production in human monocytes challenged by C. albicans. These studies provide insights into the early innate immune response induced by a clinically significant fungal pathogen. This work reveals a mechanism of cGAS- and STING-dependent type I IFN induction in response to biofilm-associated Candida albicans DNA packaged in extracellular vesicles.
白色念珠菌胞外囊泡通过 cGAS 和 STING 触发 I 型 IFN 信号传导
宿主 I 型干扰素(IFN)途径是人类真菌病原体白色念珠菌诱发炎症的主要标志。然而,在宿主防御白念珠菌的过程中激活这一途径的分子机制仍不清楚。在这里,我们揭示了缺乏环GMP-AMP合成酶(cGAS)-IFN基因刺激器(STING)通路成分的小鼠在受到白念珠菌静脉挑战后的存活率有所提高。通过诱导干扰素刺激基因、IFNβ的产生以及IFN调节因子3和TANK结合激酶1的磷酸化,可以确定包裹在细胞外囊泡中的生物膜相关白僵菌DNA触发了cGAS-STING通路。细胞外囊泡诱导的 I 型 IFNs 激活与 Dectin-1/Card9 通路无关,也不需要收费样受体 9。cGAS 和 STING 的单核苷酸多态性可有效改变受到白僵菌挑战的人类单核细胞的炎性细胞因子产生。这些研究为临床上重要的真菌病原体诱导的早期先天性免疫反应提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


