Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation
IF 25.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
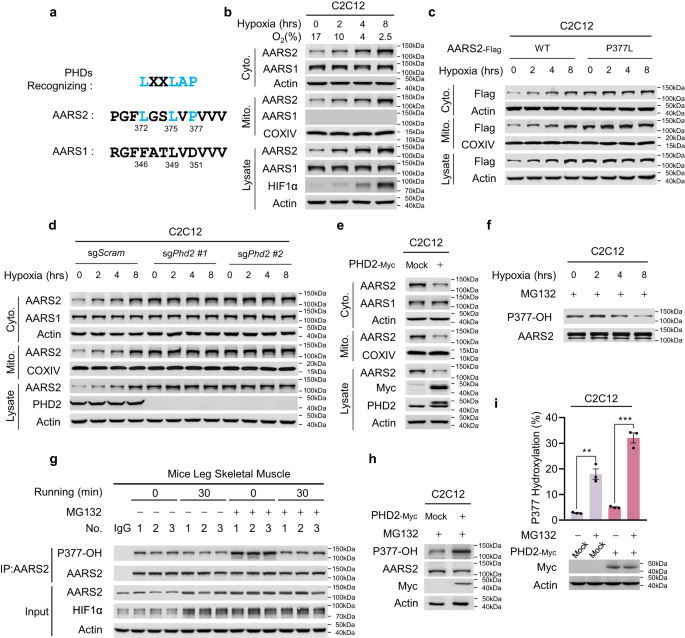
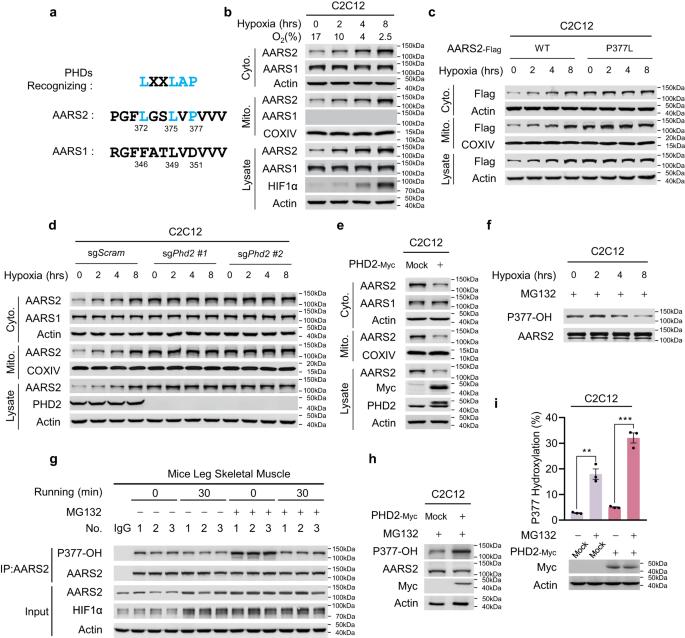
Oxidative phosphorylation (OXPHOS) consumes oxygen to produce ATP. However, the mechanism that balances OXPHOS activity and intracellular oxygen availability remains elusive. Here, we report that mitochondrial protein lactylation is induced by intracellular hypoxia to constrain OXPHOS. We show that mitochondrial alanyl-tRNA synthetase (AARS2) is a protein lysine lactyltransferase, whose proteasomal degradation is enhanced by proline 377 hydroxylation catalyzed by the oxygen-sensing hydroxylase PHD2. Hypoxia induces AARS2 accumulation to lactylate PDHA1 lysine 336 in the pyruvate dehydrogenase complex and carnitine palmitoyltransferase 2 (CPT2) lysine 457/8, inactivating both enzymes and inhibiting OXPHOS by limiting acetyl-CoA influx from pyruvate and fatty acid oxidation, respectively. PDHA1 and CPT2 lactylation can be reversed by SIRT3 to activate OXPHOS. In mouse muscle cells, lactylation is induced by lactate oxidation-induced intracellular hypoxia during exercise to constrain high-intensity endurance running exhaustion time, which can be increased or decreased by decreasing or increasing lactylation levels, respectively. Our results reveal that mitochondrial protein lactylation integrates intracellular hypoxia and lactate signals to regulate OXPHOS.
缺氧会诱导线粒体蛋白质乳化,从而限制氧化磷酸化。
氧化磷酸化(OXPHOS)消耗氧气产生 ATP。然而,平衡 OXPHOS 活性和细胞内氧气可用性的机制仍未确定。在这里,我们报告了线粒体蛋白乳酰化是由细胞内缺氧诱导来限制 OXPHOS 的。我们发现线粒体丙氨酰-tRNA 合成酶(AARS2)是一种蛋白质赖氨酸乳酰转移酶,其蛋白酶体降解在氧传感羟化酶 PHD2 催化的脯氨酸 377 羟化作用下得到加强。缺氧诱导 AARS2 积累,使丙酮酸脱氢酶复合物中的 PDHA1 336 赖氨酸和肉碱棕榈酰基转移酶 2 (CPT2) 457/8 赖氨酸发生乳酰化,从而使这两种酶失活,并分别通过限制丙酮酸和脂肪酸氧化产生的乙酰-CoA 流入来抑制 OXPHOS。SIRT3 可以逆转 PDHA1 和 CPT2 的乳化作用,从而激活 OXPHOS。在小鼠肌肉细胞中,运动时乳酸氧化诱导的细胞内缺氧会诱导乳酸化,从而限制高强度耐力跑的耗竭时间,降低或增加乳酸化水平可分别增加或减少耗竭时间。我们的研究结果揭示了线粒体蛋白乳酰化整合了细胞内缺氧和乳酸信号以调控OXPHOS。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


