Visible Light-Induced Hydroacylation of Benzylidenemalononitriles with Aroyl Chlorides Using Silane as a Hydrogen Donor
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A novel photoredox-catalyzed direct hydroacylation of benzylidenemalononitriles is described. In this method, aroyl chlorides are employed as a readily available and affordable source of acyl groups, while commercially available tris(trimethylsilyl)silane acts as both the hydrogen atom donor and electron donor. By eliminating the requirement for complex synthesis of acyl precursors and hydrogen atom-transfer (HAT) reagents, this approach offers a convenient and efficient strategy for the hydroacylation of benzylidenemalononitriles.
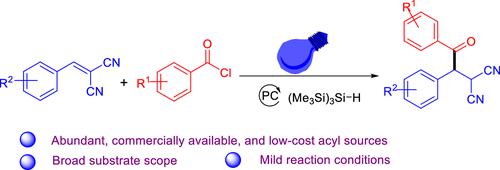
以硅烷为氢供体,利用可见光诱导亚苄基丙二腈与丙酰氯发生羟基化反应。
本文介绍了一种新型的光氧化催化苄叉丙二腈直接加氢反应。在这种方法中,酰基氯被用作酰基的现成来源,价格低廉,而市售的三(三甲基硅基)硅烷既是氢原子供体,又是电子供体。这种方法不需要复杂的酰基前体合成和氢原子转移(HAT)试剂,为苯亚甲基丙二腈的氢酰化提供了一种便捷高效的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


