Evaluation of gadolinium-based contrast agents in juvenile CD-1 mice including behavioral evaluations
Abstract
Introduction
Seven gadolinium-based contrast agents (GBCAs), four linear and three macrocyclic, were evaluated for potential effects on development, including behavior of juvenile CD-1 mice.
Methods
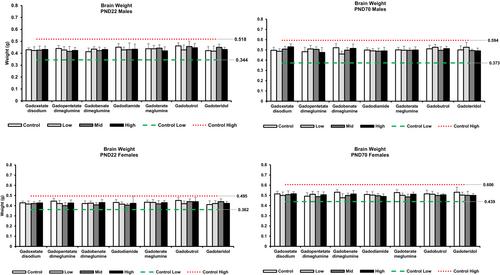
The GBCAs were administered via intravenous injection once daily on postnatal day (PND) 9, 12, 15, 18, and 21 (PND 1 was the day of delivery) at doses up to twice the human equivalent clinical dose (i.e., 0.63 mmol Gd/kg for gadoxetate disodium and 2.5 mmol Gd/kg for the other GBCAs). Mice were bled for evaluation of exposure (plasma) to gadolinium (Gd) on PND 9, 12, and 70. At scheduled euthanasia, the liver, spleen, brain, skin (dorsal surface), bone (left femur), and kidneys were excised from up to six mice/sex/group on PND 10, 22, or 70 for the determination of Gd levels and histopathological analysis. All mice were monitored for toxicity, growth and survival, sexual maturation, and behavior.
Conclusion
Gd was quantifiable in the brain tissues with levels declining over time. There was no long-term effect on the growth and development for mice exposed to any of the GBCAs. There was no impact on neurodevelopment as assessed by brain histology and validated neurobehavioral tests, including a functional observational battery, motor activity, and learning and memory as evaluated in the Morris water maze. For all GBCAs, the highest dose tested represented the no-observable-adverse-effect level in juvenile mice.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


