Asian participants' experience in phase 3/3b studies of long-acting cabotegravir and rilpivirine: Efficacy, safety, pharmacokinetic, and virological outcomes through week 96
Abstract
Objectives
Cabotegravir + rilpivirine (CAB + RPV) dosed monthly or every 2 months is the first complete long-acting (LA) regimen recommended by treatment guidelines for the maintenance of HIV-1 virological suppression. This post hoc analysis summarizes outcomes for Asian participants through week 96.
Methods
Data from Asian participants naive to CAB + RPV randomized to receive dosing every 4 weeks (Q4W) or every 8 weeks (Q8W) in the FLAIR (NCT02938520) and ATLAS-2M (NCT03299049) phase 3/3b studies were pooled. The proportion of participants with plasma HIV-1 RNA ≥50 and <50 copies/mL (per FDA Snapshot algorithm), incidence of confirmed virological failure (CVF; two consecutive HIV-1 RNA ≥200 copies/mL), pharmacokinetics, safety, and tolerability through week 96 were assessed.
Results
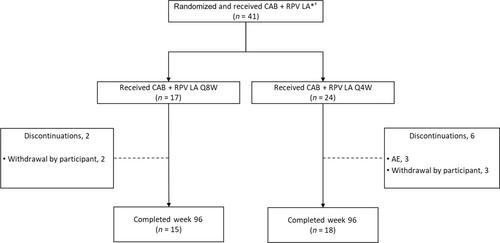
Overall, 41 Asian participants received CAB + RPV (Q8W, n = 17; Q4W, n = 24). At week 96, 83% (n = 34/41) of participants maintained HIV-1 RNA <50 copies/mL, none had HIV-1 RNA ≥50 copies/mL, and 17% (n = 7/41) had no virological data. No Asian participant met the CVF criterion. Drug-related adverse events occurred in 44% (n = 18/41) of participants; none were Grade ≥3. All injection site reactions were Grade 1 or 2; median duration was 2 days and most resolved within 7 days (90%, n = 390/435). CAB and RPV trough concentrations remained well above their respective protein-adjusted 90% inhibitory concentrations (CAB, 0.166 μg/mL; RPV, 12 ng/mL) through week 96.
Conclusions
CAB + RPV LA demonstrated high efficacy, with no participants having CVF, and an acceptable safety profile in Asian participants through week 96. These data support CAB + RPV LA as a complete regimen for the maintenance of HIV-1 virological suppression in Asian individuals.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


