Rescue of p53 functions by in vitro-transcribed mRNA impedes the growth of high-grade serous ovarian cancer
Abstract
Background
The cellular tumor protein p53 (TP53) is a tumor suppressor gene that is frequently mutated in human cancers. Among various cancer types, the very aggressive high-grade serous ovarian carcinoma (HGSOC) exhibits the highest prevalence of TP53 mutations, present in >96% of cases. Despite intensive efforts to reactivate p53, no clinical drug has been approved to rescue p53 function. In this study, our primary objective was to administer in vitro-transcribed (IVT) wild-type (WT) p53-mRNA to HGSOC cell lines, primary cells, and orthotopic mouse models, with the aim of exploring its impact on inhibiting tumor growth and dissemination, both in vitro and in vivo.
Methods
To restore the activity of p53, WT p53 was exogenously expressed in HGSOC cell lines using a mammalian vector system. Moreover, IVT WT p53 mRNA was delivered into different HGSOC model systems (primary cells and patient-derived organoids) using liposomes and studied for proliferation, cell cycle progression, apoptosis, colony formation, and chromosomal instability. Transcriptomic alterations induced by p53 mRNA were analyzed using RNA sequencing in OVCAR-8 and primary HGSOC cells, followed by ingenuity pathway analysis. In vivo effects on tumor growth and metastasis were studied using orthotopic xenografts and metastatic intraperitoneal mouse models.
Results
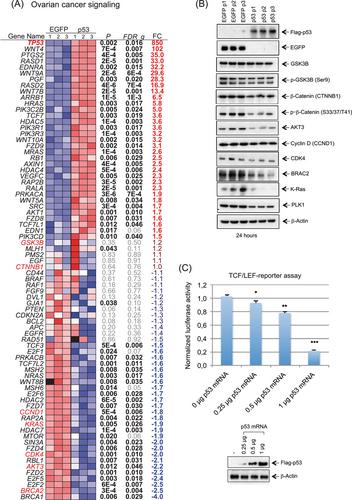
Reactivation of the TP53 tumor suppressor gene was explored in different HGSOC model systems using newly designed IVT mRNA-based methods. The introduction of WT p53 mRNA triggered dose-dependent apoptosis, cell cycle arrest, and potent long-lasting inhibition of HGSOC cell proliferation. Transcriptome analysis of OVCAR-8 cells upon mRNA-based p53 reactivation revealed significant alterations in gene expression related to p53 signaling, such as apoptosis, cell cycle regulation, and DNA damage. Restoring p53 function concurrently reduces chromosomal instability within the HGSOC cells, underscoring its crucial contribution in safeguarding genomic integrity by moderating the baseline occurrence of double-strand breaks arising from replication stress. Furthermore, in various mouse models, treatment with p53 mRNA reduced tumor growth and inhibited tumor cell dissemination in the peritoneal cavity in a dose-dependent manner.
Conclusions
The IVT mRNA-based reactivation of p53 holds promise as a potential therapeutic strategy for HGSOC, providing valuable insights into the molecular mechanisms underlying p53 function and its relevance in ovarian cancer treatment.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


