Brønsted acid-promoted synthesis of polysubstituted pyrroles from enamines/imines and diazopyruvates: A metal-free cascade approach
引用次数: 0
Abstract
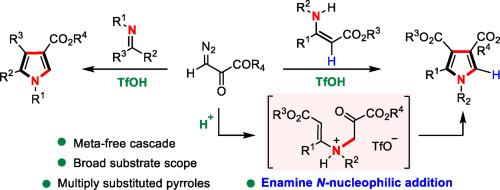
A Brønsted acid-promoted, metal-free cascade reaction of easily available enamines/imines with diazopyruvates has been demonstrated. With triflic acid as the promoter, the reaction proceeds smoothly at room temperature through sequential diazo protonation, nucleophilic enamine N-addition/C-addition and dehydrative aromatisation in a highly regioselective manner. Interestingly, the regioselectivity of the reaction is governed by the unusual enamine N-nucleophilic addition. The method provides an operationally trivial approach to the synthesis of multisubstituted pyrroles including tri-, tetra-, and penta-substituted derivatives as well as N–H free pyrroles with diverse functionalities in good yields under extraordinarily simple and mild conditions. The utility of the method is illustrated by the rapid assembly of polysubstituted bispyrroles and an array of diversely structured pyrrole derivatives.

在布氏酸的促进下,从烯胺/亚胺和重氮丙酮酸盐合成多代吡咯:一种无金属级联方法
研究人员展示了一种由勃氏酸促进的、不含金属的级联反应,该反应是将易于获得的烯胺/亚胺与重氮丙酮酸盐进行反应。以三氟丙烯酸为促进剂,该反应在室温下以高度区域选择性的方式,通过重氮质子化、亲核烯胺 N-加成/C-加成和脱水芳香化的顺序顺利进行。有趣的是,该反应的区域选择性是由不寻常的烯胺 N-亲核加成决定的。该方法为合成多取代吡咯(包括三、四和五取代衍生物)以及具有不同官能度的 N-H 游离吡咯提供了一种操作简便的方法,而且条件异常简单温和。通过快速组装多取代双吡咯和一系列结构多样的吡咯衍生物,说明了该方法的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


