Pharmacological reactivation of p53 in the era of precision anticancer medicine
IF 81.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
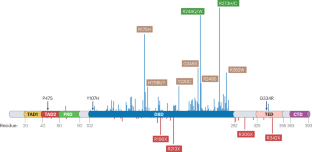
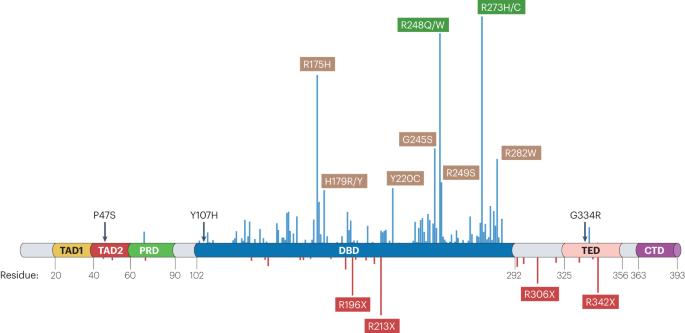
p53, which is encoded by the most frequently mutated gene in cancer, TP53, is an attractive target for novel cancer therapies. Despite major challenges associated with this approach, several compounds that either augment the activity of wild-type p53 or restore all, or some, of the wild-type functions to p53 mutants are currently being explored. In wild-type TP53 cancer cells, p53 function is often abrogated by overexpression of the negative regulator MDM2, and agents that disrupt p53–MDM2 binding can trigger a robust p53 response, albeit potentially with induction of p53 activity in non-malignant cells. In TP53-mutant cancer cells, compounds that promote the refolding of missense mutant p53 or the translational readthrough of nonsense mutant TP53 might elicit potent cell death. Some of these compounds have been, or are being, tested in clinical trials involving patients with various types of cancer. Nonetheless, no p53-targeting drug has so far been approved for clinical use. Advances in our understanding of p53 biology provide some clues as to the underlying reasons for the variable clinical activity of p53-restoring therapies seen thus far. In this Review, we discuss the intricate interactions between p53 and its cellular and microenvironmental contexts and factors that can influence p53’s activity. We also propose several strategies for improving the clinical efficacy of these agents through the complex perspective of p53 functionality. p53, encoded by TP53, the commonest mutated gene in cancer, is an appealing target for systemic anticancer therapies including those designed to restore p53 function. Thus far, and despite promising preclinical data and several clinical trials, no p53-restoring systemic therapy has been approved for therapeutic use. Despite this limited success, several research efforts are ongoing. In this Review, the authors summarize the role of p53 in cancer with a focus on the complexity of p53 function and how this relates to clinical attempts to restore at least some of these functions.
精准抗癌时代的 p53 药物再激活
p53是由癌症中最常见的突变基因TP53编码的,是一种有吸引力的新型癌症治疗靶点。尽管这种方法存在重大挑战,但目前正在探索几种增强野生型p53活性或恢复p53突变体全部或部分野生型功能的化合物。在野生型TP53癌细胞中,p53的功能经常被负调节因子MDM2的过度表达所破坏,而破坏p53 - MDM2结合的药物可以触发强烈的p53反应,尽管在非恶性细胞中可能会诱导p53活性。在TP53突变的癌细胞中,促进错义突变体p53的重折叠或无义突变体TP53的翻译解读的化合物可能会导致细胞死亡。其中一些化合物已经或正在进行各种癌症患者的临床试验。尽管如此,迄今为止还没有一种靶向p53的药物被批准用于临床。我们对p53生物学的理解的进步为迄今为止所见的p53恢复疗法的临床活性变化的潜在原因提供了一些线索。在这篇综述中,我们讨论了p53与其细胞和微环境背景以及影响p53活性的因素之间复杂的相互作用。我们还从p53功能的复杂角度提出了提高这些药物临床疗效的几种策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


