On the Rheology of Thixotropic and Rheopexic Suspensions
IF 16.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
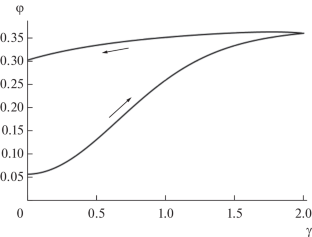
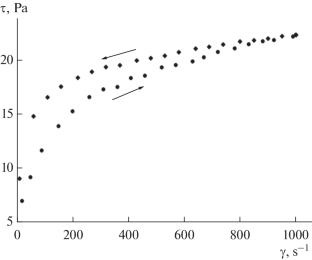
Semi-empirical equations are derived that describe the dependence of shear stress on shear rate during the flow of a one-component suspension. The suspension is considered as consisting of two fractions: single grains of the solid phase and their dimers, between which a reversible dimerization reaction occurs. In this case, the dimerization of single grains is considered as a reaction with an invariable rate constant, and the dissociation of dimers is considered as an inverse reaction with a rate constant that increases linearly with the shear rate. The equations are based on the Krieger−Doherty formula, generalized to the case of a multicomponent suspension.
关于触变悬浮液和流变悬浮液的流变学
摘要__ 本文推导出半经验方程,描述了单组分悬浮液流动过程中剪切应力与剪切速率的关系。该悬浮液被认为由两部分组成:固相的单颗粒及其二聚体,它们之间发生可逆的二聚反应。在这种情况下,单颗粒的二聚反应被认为是一种速率常数不变的反应,而二聚体的解离则被认为是一种速率常数随剪切速率线性增加的逆反应。计算公式基于 Krieger-Doherty 公式,并将其推广到多组分悬浮液中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Accounts of Chemical Research
化学-化学综合
CiteScore
31.40
自引率
1.10%
发文量
312
审稿时长
2 months
期刊介绍:
Accounts of Chemical Research presents short, concise and critical articles offering easy-to-read overviews of basic research and applications in all areas of chemistry and biochemistry. These short reviews focus on research from the author’s own laboratory and are designed to teach the reader about a research project. In addition, Accounts of Chemical Research publishes commentaries that give an informed opinion on a current research problem. Special Issues online are devoted to a single topic of unusual activity and significance.
Accounts of Chemical Research replaces the traditional article abstract with an article "Conspectus." These entries synopsize the research affording the reader a closer look at the content and significance of an article. Through this provision of a more detailed description of the article contents, the Conspectus enhances the article's discoverability by search engines and the exposure for the research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


