Calcium isotope fractionation during melt immiscibility and carbonatite petrogenesis
IF 3.7
1区 地球科学
Q1 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
Abstract
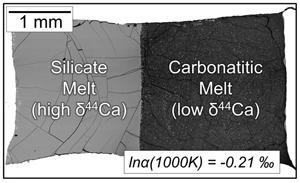
Stable calcium isotopes have been used to suggest that subducted marine carbonates are frequently involved in the formation of carbonatites. Significant Ca isotope fractionations during carbonatite petrogenesis, however, could lead to a dramatically different picture. We present Ca isotope data for (i) coexisting (immiscible) carbonatite and silicate melts from high temperature centrifuging piston cylinder experiments, (ii) primary apatite and calcite/dolomite from natural carbonatites, and (iii) ab initio estimates for equilibrium Ca isotope partitioning in calcite, dolomite, and ankerite. Carbonatitic melts have lower δ44Ca than their conjugate silicate melts, with an equilibrium fractionation factor [1000lnα(1000K)] of −0.21 ± 0.06 (tSE). We develop a quantitative four stage model for carbonatite petrogenesis (partial melting followed by fractional crystallisation, silicate-carbonatite melt immiscibility, and calcite/apatite accumulation) that fully explains our natural data (average δ44CaBSE of −0.30 ± 0.03 ‰) and those from recent studies, without requiring isotopic contributions from recycled marine carbonates. Our results suggest that lighter isotopes of similarly bound cations (e.g., Mg, Fe, Sr, Ba, Zn) should be preferentially incorporated into carbonatitic melts and that calciocarbonatite formation involves melt immiscibility after differentiation of mantle-derived alkaline CO2-bearing silicate melts.
熔体不混溶与碳酸盐岩成岩过程中的钙同位素分馏
稳定的钙同位素表明,俯冲的海相碳酸盐岩经常参与碳酸盐岩的形成。然而,在碳酸盐岩岩石形成过程中,显著的Ca同位素分馏可能导致一个截然不同的画面。我们给出了(i)高温离心活塞缸实验中共存(不混溶)碳酸盐和硅酸盐熔体的Ca同位素数据,(ii)天然碳酸盐中的原生磷灰石和方解石/白云石,以及(iii)从头计算方解石、白云石和铁白云石中平衡Ca同位素分配的估计。碳酸盐岩熔体的δ44Ca低于其共轭硅酸盐熔体,平衡分馏因子[1000lnα(1000K)]为−0.21±0.06 (tSE)。我们建立了一个定量的碳酸盐岩岩石成因四阶段模型(部分熔融后是部分结晶,硅酸盐-碳酸盐岩熔体不混溶,方解石/磷灰石聚集),该模型完全解释了我们的自然数据(平均δ44CaBSE为- 0.30±0.03‰)和最近研究的数据,而不需要来自回收海相碳酸盐岩的同位素贡献。我们的研究结果表明,类似结合阳离子的较轻同位素(如Mg, Fe, Sr, Ba, Zn)应该优先被合并到碳酸盐岩熔体中,并且钙碳酸盐的形成涉及地幔源碱性含二氧化碳硅酸盐熔体分化后的熔体不混溶。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Geochemical Perspectives Letters
Earth and Planetary Sciences-Geochemistry and Petrology
CiteScore
7.00
自引率
2.00%
发文量
42
审稿时长
15 weeks
期刊介绍:
Geochemical Perspectives Letters is an open access, internationally peer-reviewed journal of the European Association of Geochemistry (EAG) that publishes short, highest-quality articles spanning geochemical sciences. The journal aims at rapid publication of the most novel research in geochemistry with a focus on outstanding quality, international importance, originality, and stimulating new developments across the vast array of geochemical disciplines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


