Intentional corrosion-induced reconstruction of defective NiFe layered double hydroxide boosts electrocatalytic nitrate reduction to ammonia
引用次数: 0
Abstract
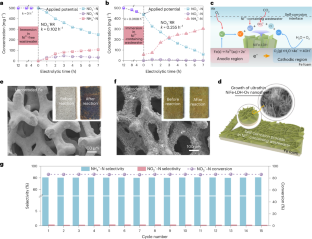
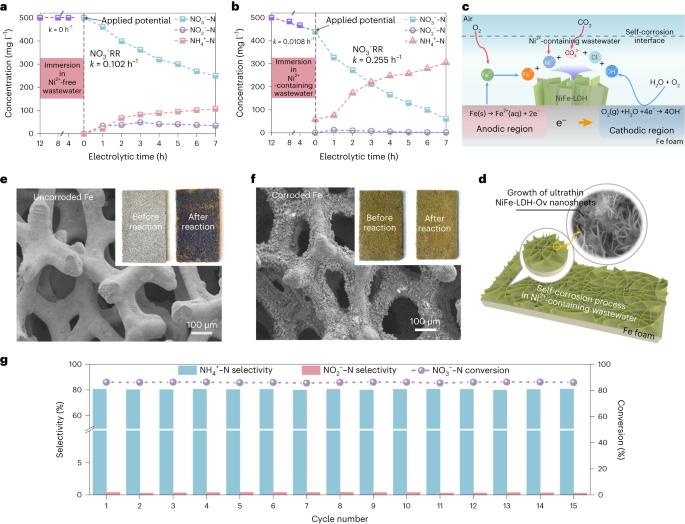
The electroreduction of nitrate to ammonia is particularly important in mitigating environmental pollution and obtaining value-added products. Although non-toxic and inexpensive iron-based materials are expected to be a promising catalyst for electrochemical nitrate reduction, ensuring their sustained high activity and inhibiting spontaneous corrosion requires the implementation of complex design. Here we report an economical self-corrosion approach that utilizes Ni2+ ions in wastewater to control the formation of NiFe layered double hydroxide active phase on iron surface, resulting in high nitrate conversion (97.2%) and ammonia selectivity (90.3%). Coupling nitrate reduction with acid absorption, the conversion from NO3− to (NH4)2SO4(s) for applications such as acting as fertilizer are achieved. This distinctive ‘waste-to-treasure’ perspective not only challenges the conventional belief that corrosion diminishes active phase but also notably improves catalytic efficiency while harnessing valuable resources from wastewater, offering a practical method for converting nitrate to useful ammonia products. Fe-based electrodes emerge as an effective and economical option to treat nitrate-laden wastewater. Whereas Fe cathode corrosion is commonly considered as an adverse factor for the electroreduction of nitrate to ammonia, intentional corrosion-induced surficial reconstruction has the potential to enhance catalytic performance.
有缺陷的NiFe层状双氢氧化物的故意腐蚀诱导重建促进了电催化硝酸还原为氨
硝酸电还原制氨对减轻环境污染和获得高附加值产品尤为重要。虽然无毒、廉价的铁基材料有望成为一种很有前途的电化学硝酸还原催化剂,但要确保其持续的高活性和抑制自发腐蚀,需要实施复杂的设计。本文报道了一种经济的自腐蚀方法,该方法利用废水中的Ni2+离子控制铁表面NiFe层状双氢氧化物活性相的形成,从而获得高硝酸盐转化率(97.2%)和氨选择性(90.3%)。将硝酸盐还原与酸吸收相结合,实现了NO3−转化为(NH4)2SO4的应用,如作为肥料。这种独特的“废物转化为宝藏”的观点不仅挑战了腐蚀会减少活性相的传统观点,而且在利用废水宝贵资源的同时显著提高了催化效率,为将硝酸盐转化为有用的氨产品提供了一种实用的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


