Identification of elongation factor-2 as a novel regulator of mitochondrial fission
引用次数: 0
Abstract
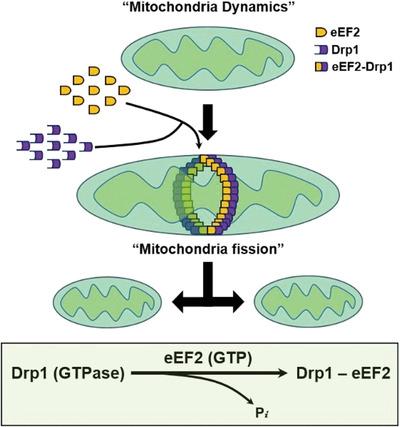
Mitochondria continuously undergo morphologically dynamic processes of fusion and fission to maintain their size, shape, amount, and function; yet the precise molecular mechanisms by which mitochondrial dynamics is regulated remain to be fully elucidated. Here, we report a previous unappreciated but critical role of eukaryotic elongation factor 2 (eEF2) in regulating mitochondrial fission. eEF2, a G-protein superfamily member encoded by EEF2 gene in humans, has long been appreciated as a promoter of the GTP-dependent translocation of the ribosome during protein synthesis. We found unexpectedly in several types of cells that eEF2 was not only present in the cytosol but also in the mitochondria. Furthermore, we showed that mitochondrial length was significantly increased when the cells were subjected to silencing of eEF2 expression, suggesting a promotive role for eEF2 in the mitochondrial fission. Inversely, overexpression of eEF2 decreased mitochondrial length, suggesting an increase of mitochondrial fission. Inhibition of mitochondrial fission caused by eEF2 depletion was accompanied by alterations of cellular metabolism, as evidenced by a reduction of oxygen consumption and an increase of oxidative stress in the mitochondria. We further demonstrated that eEF2 and Drp1, a key driver of mitochondrial fission, colocalized at the mitochondria, as evidenced by microscopic observation, coimmunoprecipitation, and GST pulldown assay. Deletion of the GTP-binding motif of eEF2 decreased its association with Drp1 and abrogated its effect on mitochondria fission. Moreover, we showed that wild-type eEF2 stimulated GTPase activity of Drp1, whereas deletion of the GTP-binding site of eEF2 diminished its stimulatory effect on GTPase activity. This work not only reveals a previously unrecognized function of eEF2 (i.e., promoting mitochondrial fission), but also uncovers the interaction of eEF2 with Drp1 as a novel regulatory mechanism of the mitochondrial dynamics. Therefore, eEF2 warrants further exploration for its potential as a therapeutic target for the mitochondria-related diseases.
延长因子-2作为线粒体裂变新调控因子的鉴定
线粒体不断经历形态动态的融合和裂变过程,以维持其大小、形状、数量和功能;然而,线粒体动力学调控的精确分子机制仍有待充分阐明。在这里,我们报告了以前未被认识但关键的真核延伸因子2 (eEF2)在调节线粒体裂变中的作用。eEF2是由人类eEF2基因编码的g蛋白超家族成员,长期以来一直被认为是蛋白质合成过程中核糖体gtp依赖性易位的启动子。我们意外地发现,在几种类型的细胞中,eEF2不仅存在于细胞质中,而且存在于线粒体中。此外,我们发现当细胞沉默eEF2表达时,线粒体长度显着增加,这表明eEF2在线粒体裂变中起促进作用。相反,过表达eEF2减少了线粒体长度,表明线粒体分裂增加。eEF2耗竭引起的线粒体裂变抑制伴随着细胞代谢的改变,线粒体中氧气消耗的减少和氧化应激的增加证明了这一点。通过显微镜观察、共免疫沉淀和GST下拉实验,我们进一步证明了eEF2和Drp1是线粒体分裂的关键驱动因素,它们共定位于线粒体。eEF2的gtp结合基序的缺失降低了其与Drp1的关联,并取消了其对线粒体裂变的影响。此外,我们发现野生型eEF2刺激了Drp1的GTPase活性,而eEF2的gtp结合位点的缺失减少了其对GTPase活性的刺激作用。这项工作不仅揭示了eEF2以前未被认识的功能(即促进线粒体裂变),而且还揭示了eEF2与Drp1的相互作用是线粒体动力学的一种新的调节机制。因此,eEF2作为线粒体相关疾病的治疗靶点的潜力值得进一步探索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


