Ferric nitrate-catalyzed aerobic oxidative ring-opening of substituted furans for the stereoselective synthesis of (Z)-1,4-enediones
引用次数: 0
Abstract
A simple and highly efficient catalytic system for the selective aerobic oxidative ring-opening of substituted furans has been achieved using Fe(NO3)3·9H2O as a catalyst and air as an oxidant under mild conditions. A series of (Z)-1,4-enediones were obtained in good yields (up to 97 %) with excellent stereoselectivity (up to > 20:1 Z/E ratio). The present synthetic method exhibits perfect atom economy, wide substrate scope, and highly functionalized products allowing diverse transformations.
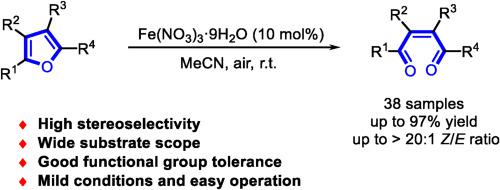

硝酸铁催化取代呋喃的有氧氧化开环立体选择性合成(Z)-1,4-烯二酮
在温和条件下,以Fe(NO3)3·9H2O为催化剂,空气为氧化剂,实现了一种简单高效的取代呋喃选择性好氧开环催化体系。得到一系列(Z)-1,4-烯二酮,产率高达97%,具有良好的立体选择性(高达>Z/E比20:1)。本合成方法具有原子经济性好、底物范围广、产品功能化程度高、可进行多种转化等特点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


