Sorption of water and ethanol pure vapours and vapour mixtures by four hardwoods
Abstract
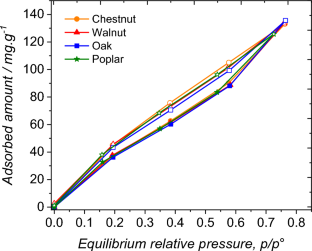
Water is a universal solvent and swelling agent that is widely used in wood industry in association with organic solvents and salts, whether for the fractionation of biomass and the production of bio-based synthons for the chemical industry, the application of sizing agents and painting for the paper industry or the incorporation of preservatives to enhance wood durability for the timber industry. The relevance of solvents and technical treatments used for wood-based products requires a proper identification of the specific role of each solvent on wood biopolymers to better understand and predict their influence on wood properties. In particular, wood impregnated with aqueous solutions of organic solvents has shown to give rise to greater swelling than that observed in pure water, described as “hyperswelling”. To understand this phenomenon, the first step is to examine the existing interactions between wood microstructure and the different solvents present in these mixtures. This study is an attempt to bring to light the sorption behaviour of four different hardwoods in water–ethanol vapour mixtures containing increasing molar fractions of ethanol from 0 to 100%. Contrasted sorption behaviour in pure solvents was observed according to wood species having different biochemical compositions. This behaviour highlights the different affinities of ethanol and water for the macromolecules present in the wood microstructure. With mixed solvents, peculiar effects were confirmed in sorption behaviour of woods with lower mixed solvent uptake at high partial pressures compared to pure solvents. It is also shown that part of the sorbed ethanol molecules remains chemisorbed in the wood structures at the end of the desorption process.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


