A human embryonic limb cell atlas resolved in space and time
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Human limbs emerge during the fourth post-conception week as mesenchymal buds, which develop into fully formed limbs over the subsequent months1. This process is orchestrated by numerous temporally and spatially restricted gene expression programmes, making congenital alterations in phenotype common2. Decades of work with model organisms have defined the fundamental mechanisms underlying vertebrate limb development, but an in-depth characterization of this process in humans has yet to be performed. Here we detail human embryonic limb development across space and time using single-cell and spatial transcriptomics. We demonstrate extensive diversification of cells from a few multipotent progenitors to myriad differentiated cell states, including several novel cell populations. We uncover two waves of human muscle development, each characterized by different cell states regulated by separate gene expression programmes, and identify musculin (MSC) as a key transcriptional repressor maintaining muscle stem cell identity. Through assembly of multiple anatomically continuous spatial transcriptomic samples using VisiumStitcher, we map cells across a sagittal section of a whole fetal hindlimb. We reveal a clear anatomical segregation between genes linked to brachydactyly and polysyndactyly, and uncover transcriptionally and spatially distinct populations of the mesenchyme in the autopod. Finally, we perform single-cell RNA sequencing on mouse embryonic limbs to facilitate cross-species developmental comparison, finding substantial homology between the two species. Using single-cell and spatial transcriptomics, human embryonic limb development across space and time and the diversification and cross-species conservation of cells are demonstrated.
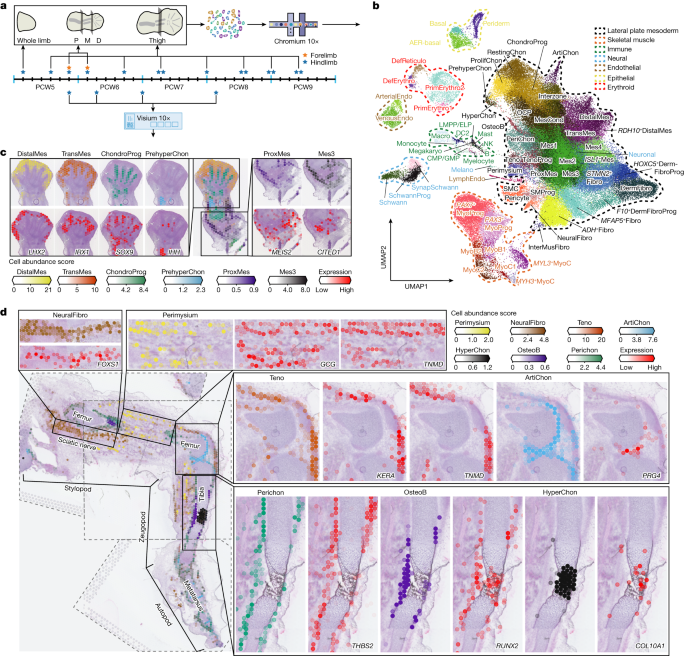

人类胚胎肢体细胞图谱在空间和时间上的解析。
人的四肢在受孕后的第四个星期以间充质芽的形式出现,在随后的几个月里发育成完全成形的四肢。这一过程是由许多受时间和空间限制的基因表达程序精心策划的,使得表型的先天性改变很常见。几十年来,对模式生物的研究已经确定了脊椎动物肢体发育的基本机制,但尚未对人类这一过程进行深入的表征。在这里,我们使用单细胞和空间转录组学详细介绍了人类胚胎肢体在空间和时间上的发育。我们证明了细胞从几种多能祖细胞到无数种分化细胞状态的广泛多样化,包括几种新的细胞群体。我们发现了人类肌肉发育的两波,每波都以不同的细胞状态为特征,由不同的基因表达程序调节,并确定了肌肉蛋白(MSC)是维持肌肉干细胞身份的关键转录抑制因子。通过使用VisiumStitcher组装多个解剖上连续的空间转录组样本,我们绘制了整个胎儿后肢矢状切片的细胞图谱。我们揭示了与短指和多指相关的基因在解剖学上的明显分离,并揭示了自足动物中间叶质在转录和空间上的不同群体。最后,我们对小鼠胚胎肢体进行单细胞RNA测序,以便进行跨物种发育比较,发现两个物种之间存在大量同源性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


