An efficient, concise synthetic approach to γ-aminobutyric acid (GABA) derivatives bearing bicyclo[2.2.2]octane and bicyclo[2.2.1]heptane rings
Abstract
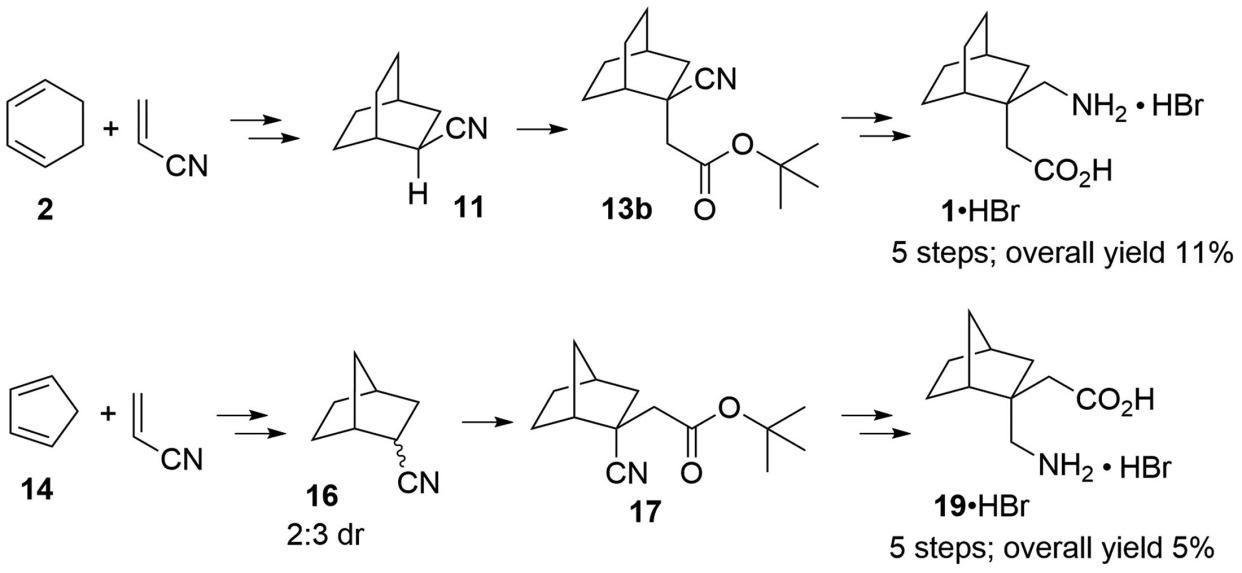
An efficient, concise 5-step synthetic approach to γ-aminobutyric acid (GABA) derivatives bearing bridged bicyclo[2.2.2]octane and bicyclo[2.2.1]heptane rings was developed, which consists of Diels-Alder cycloaddition to construct the bridged bicyclic rings with a nitrile functionality, hydrogenation, LDA-mediated SN2 alkylation to introduce acetate moiety at the α-position of nitrile, reduction of the nitrile functionality to amino group followed by intramolecular formation of lactam and finally acidic hydrolysis of lactam to give the desired GABA derivatives (1 and 19 as HBr salts). The reaction conditions of Diels-Alder cycloaddition and LDA-mediated SN2 alkylation were intensively optimized to improve the yields. The stereochemistry of the SN2 reaction involved in the bicyclo[2.2.1]heptane ring system was unambiguously elucidated by single-crystal X-ray diffraction. This synthetic approach possesses advantages of less reaction steps, high overall yields and avoidance of toxic reagents, and may find wide applications in the synthesis of other GABA derivatives with similar bicyclic or polycyclic rings.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


