Disease-specific tau filaments assemble via polymorphic intermediates
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
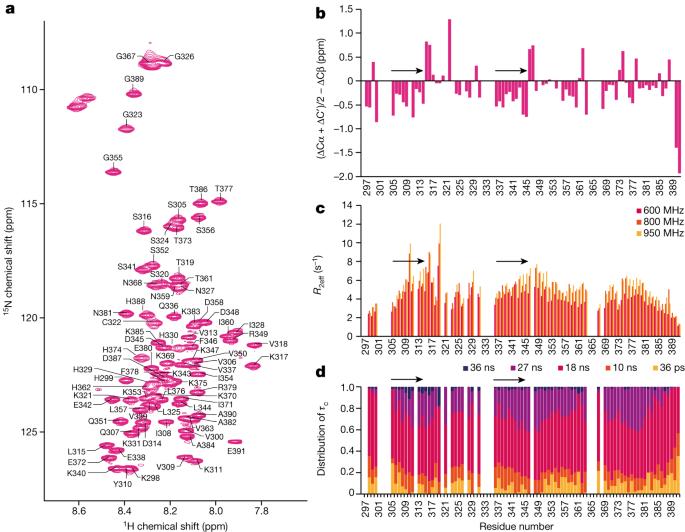
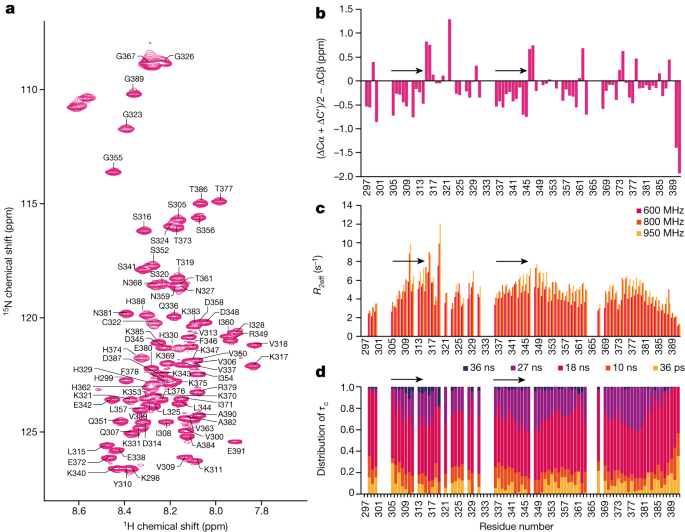
Intermediate species in the assembly of amyloid filaments are believed to play a central role in neurodegenerative diseases and may constitute important targets for therapeutic intervention1,2. However, structural information about intermediate species has been scarce and the molecular mechanisms by which amyloids assemble remain largely unknown. Here we use time-resolved cryogenic electron microscopy to study the in vitro assembly of recombinant truncated tau (amino acid residues 297–391) into paired helical filaments of Alzheimer’s disease or into filaments of chronic traumatic encephalopathy3. We report the formation of a shared first intermediate amyloid filament, with an ordered core comprising residues 302–316. Nuclear magnetic resonance indicates that the same residues adopt rigid, β-strand-like conformations in monomeric tau. At later time points, the first intermediate amyloid disappears and we observe many different intermediate amyloid filaments, with structures that depend on the reaction conditions. At the end of both assembly reactions, most intermediate amyloids disappear and filaments with the same ordered cores as those from human brains remain. Our results provide structural insights into the processes of primary and secondary nucleation of amyloid assembly, with implications for the design of new therapies. A time-resolved cryogenic electron microscopy analysis provides structural information on the processes of primary and secondary nucleation of tau amyloid formation, with implications for the development of new therapies.
疾病特异性tau蛋白丝通过多态中间体组装。
淀粉样蛋白细丝组装中的中间物质被认为在神经退行性疾病中起着核心作用,并可能构成治疗干预的重要靶点1,2。然而,关于中间物种的结构信息很少,淀粉样蛋白组装的分子机制在很大程度上仍然未知。在这里,我们使用时间分辨率低温电子显微镜研究了重组截断的tau(氨基酸残基297-391)在体外组装到成对的阿尔茨海默病螺旋细丝或慢性创伤性脑病细丝3。我们报告了一个共享的第一中间淀粉样蛋白细丝的形成,具有包含残基302-316的有序核心。核磁共振表明,相同的残基在单体tau中采用刚性的β-链状构象。在稍后的时间点,第一个中间淀粉样蛋白消失,我们观察到许多不同的中间淀粉样蛋白细丝,其结构取决于反应条件。在这两种组装反应结束时,大多数中间淀粉样蛋白消失,而具有与人类大脑相同有序核的细丝保留了下来。我们的研究结果为淀粉样蛋白组装的初级和次级成核过程提供了结构性的见解,对新疗法的设计具有启示意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


