Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets
IF 122.7
1区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
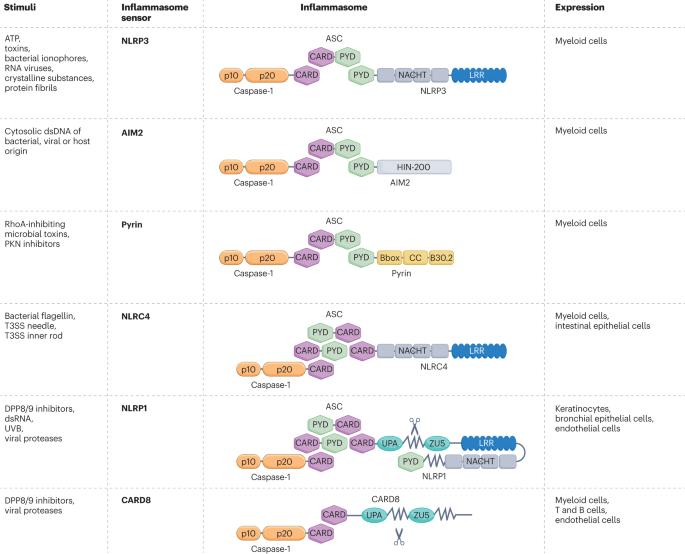
Diseases associated with chronic inflammation constitute a major health burden across the world. As central instigators of the inflammatory response to infection and tissue damage, inflammasomes — and the NACHT, LRR and PYD domain-containing protein 3 (NLRP3) inflammasome in particular — have emerged as key regulators in diverse rheumatic, metabolic and neurodegenerative diseases. Similarly to other inflammasome sensors, NLRP3 assembles a cytosolic innate immune complex that activates the cysteine protease caspase-1, which in turn cleaves gasdermin D (GSDMD) to induce pyroptosis, a regulated mode of lytic cell death. Pyroptosis is highly inflammatory, partly because of the concomitant extracellular release of the inflammasome-dependent cytokines IL-1β and IL-18 along with a myriad of additional danger signals and intracellular antigens. Here, we discuss how NLRP3 and downstream inflammasome effectors such as GSDMD, apoptosis-associated speck-like protein containing a CARD (ASC) and nerve injury-induced protein 1 (NINJ1) have gained significant traction as therapeutic targets. We highlight the recent progress in developing small-molecule and biologic inhibitors that are advancing into the clinic and serving to harness the broad therapeutic potential of modulating the NLRP3 inflammasome. Inflammasomes are central instigators of the inflammatory response to infection and tissue damage and key regulators in diverse diseases. Here, the authors describe signalling mechanisms that regulate the NLRP3 inflammasome pathways and recent progress in the development of inhibitors and agonists that are advancing into the clinic.
NLRP3炎性体的药物治疗:从信号机制到治疗靶点
与慢性炎症相关的疾病在全世界构成了一个主要的健康负担。炎症小体,尤其是NACHT、LRR和PYD结构域蛋白3 (NLRP3)炎症小体,作为感染和组织损伤炎症反应的中心促动者,已成为多种风湿性、代谢性和神经退行性疾病的关键调节因子。与其他炎性小体传感器类似,NLRP3组装胞质内先天免疫复合物,激活半胱氨酸蛋白酶caspase-1,进而裂解气皮蛋白D (GSDMD)诱导焦亡,这是一种受调节的溶解性细胞死亡模式。焦亡是高度炎症性的,部分原因是伴随炎性小体依赖性细胞因子IL-1β和IL-18的细胞外释放,以及无数额外的危险信号和细胞内抗原。在这里,我们讨论了NLRP3和下游炎性体效应物如GSDMD、含有CARD的凋亡相关斑点样蛋白(ASC)和神经损伤诱导蛋白1 (NINJ1)如何作为治疗靶点获得显著的关注。我们重点介绍了最近在开发小分子和生物抑制剂方面取得的进展,这些抑制剂正在进入临床,并有助于利用调节NLRP3炎症小体的广泛治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews. Drug Discovery
医学-生物工程与应用微生物
CiteScore
137.40
自引率
0.30%
发文量
227
期刊介绍:
Nature Reviews Drug Discovery is a monthly journal aimed at everyone working in the drug discovery and development arena.
Each issue includes:
Highest-quality reviews and perspectives covering a broad scope.
News stories investigating the hottest topics in drug discovery.
Timely summaries of key primary research papers.
Concise updates on the latest advances in areas such as new drug approvals, patent law, and emerging industry trends and strategies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


