Regioselective synthesis of 3H-Pyrazoles bearing difluoromethyl phosphonate group
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
A catalyst- and solvent-free 1,3-dipolar cycloaddition of a bench-stable diazo reagent, 2-diazo-1,1,3,3,3-pentafluoropropyl phosphonate with selected alkynes is reported. As a result, a series of novel 3H-pyrazoles bearing difluoromethyl phosphonate unit is obtained in moderate-to-excellent yields. This approach provides an efficient and easy route to this valuable class of compounds.
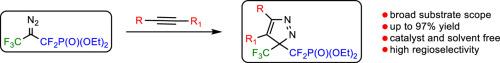
含膦酸二氟甲基3h -吡唑的区域选择性合成
报道了一种稳定的重氮试剂- 2-重氮-1,1,3,3,3-五氟丙基膦酸盐与选定的炔烃的无催化剂和无溶剂的1,3-偶极环加成反应。结果,以中等至优异的收率得到了一系列含有膦酸二氟甲基的新型3h吡唑。这种方法为这类有价值的化合物提供了一种有效而简单的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


