Co(CN)3 catalysts with well-defined coordination structure for the oxygen reduction reaction
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Metal-nitrogen-doped carbon (M-N-C) catalysts are promising platinum group metals alternatives for fuel cell cathodes; however, their randomly formed, unpredictable coordinations complicate any structure–property investigation and lead to low active site density. Here we achieve dense and well-defined cobalt sites on the surfaces of Co(CN)3 microcrystals, which are identified by single-crystal X-ray diffraction and X-ray absorption spectroscopy. In situ infrared spectroscopy and density functional theory calculations elucidate that the high oxygen reduction reaction performance (half-wave potential is 0.90 V versus RHE) of the cubic Co(CN)3 is due to its tailored coordination environment that optimizes *OH desorption. The cyanide linkage with minimized spacing between cobalt atoms maximizes active sites’ spatial density, which boosts the anion-exchange membrane fuel cell peak power density to 1.67 W cm−2. The microcrystals with well-defined coordination structures can be used as Co-N-C analogues for their structure–property relationship investigations. Anion-exchange membrane fuel cells are promising devices to produce electricity from green hydrogen, but the development of suitable cathode catalysts is required for their successful deployment. Now, Co(CN)3 microcrystals with cyanide linkages and well-defined coordination structures are shown to exhibit high oxygen reduction reaction performance in alkaline conditions.
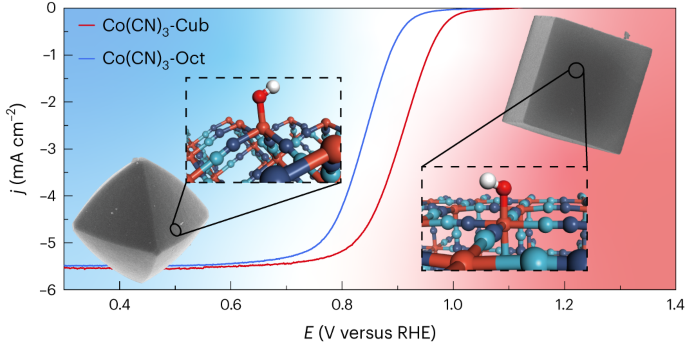
配位结构明确的Co(CN)3氧还原催化剂
金属氮掺杂碳(M-N-C)催化剂是很有前途的燃料电池阴极铂族金属替代品;然而,它们随机形成的、不可预测的配位使任何结构性质研究复杂化,并导致活性位点密度低。通过单晶x射线衍射和x射线吸收光谱分析,我们在Co(CN)3微晶表面发现了致密而清晰的钴位点。原位红外光谱和密度泛函理论计算表明,立方Co(CN)3的高氧还原反应性能(半波电位相对于RHE为0.90 V)是由于其精心设计的配位环境优化了*OH脱附。具有最小钴原子间距的氰化物键使活性位点的空间密度最大化,使阴离子交换膜燃料电池的峰值功率密度达到1.67 W cm−2。具有明确配位结构的微晶体可以作为Co-N-C类似物用于研究其结构-性能关系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


