Multiple micronutrient deficiencies in early life cause multi-kingdom alterations in the gut microbiome and intrinsic antibiotic resistance genes in mice
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
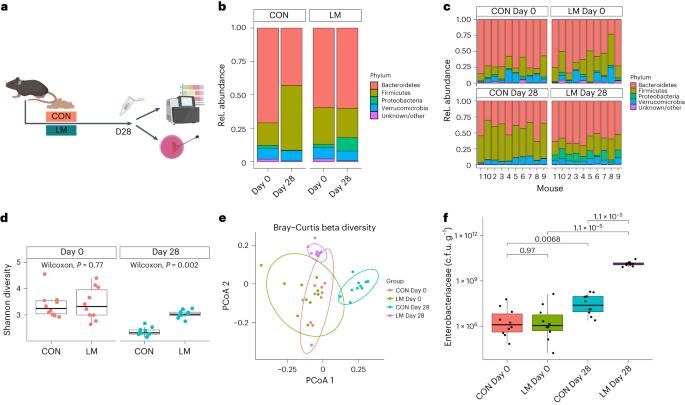
Globally, ~340 million children suffer from multiple micronutrient deficiencies, accompanied by high pathogenic burden and death due to multidrug-resistant bacteria. The microbiome is a reservoir of antimicrobial resistance (AMR), but the implications of undernutrition on the resistome is unclear. Here we used a postnatal mouse model that is deficient in multiple micronutrients (that is, zinc, folate, iron, vitamin A and vitamin B12 deficient) and shotgun metagenomic sequencing of faecal samples to characterize gut microbiome structure and functional potential, and the resistome. Enterobacteriaceae were enriched in micronutrient-deficient mice compared with mice fed an isocaloric experimental control diet. The mycobiome and virome were also altered with multiple micronutrient deficiencies including increased fungal pathogens such as Candida dubliniensis and bacteriophages. Despite being antibiotic naïve, micronutrient deficiency was associated with increased enrichment of genes and gene networks encoded by pathogenic bacteria that are directly or indirectly associated with intrinsic antibiotic resistance. Bacterial oxidative stress was associated with intrinsic antibiotic resistance in these mice. This analysis reveals multi-kingdom alterations in the gut microbiome as a result of co-occurring multiple micronutrient deficiencies and the implications for antibiotic resistance. A postnatal multiple micronutrient deficiency mouse model reveals shifts in bacterial, fungal and viral components of the gut microbiome with implications for microbiome-encoded intrinsic antibiotic resistance mechanisms.
生命早期多种微量营养素缺乏导致小鼠肠道微生物组和内在抗生素耐药基因的多王国改变。
在全球范围内,约3.4亿儿童患有多种微量营养素缺乏症,并伴有高致病性负担和多重耐药细菌导致的死亡。微生物组是抗菌素耐药性(AMR)的储存库,但营养不良对抵抗组的影响尚不清楚。在这里,我们使用了一个缺乏多种微量营养素(即锌、叶酸、铁、维生素a和维生素B12缺乏)的产后小鼠模型,并对粪便样本进行鸟枪宏基因组测序,以表征肠道微生物群结构和功能潜力,以及抵抗组。肠道杆菌科细菌在微量营养素缺乏的小鼠体内富集,与喂食等热量实验对照饮食的小鼠相比。真菌组和病毒组也因多种微量营养素缺乏而改变,包括真菌病原体如dubliniensis和噬菌体的增加。尽管是抗生素naïve,微量营养素缺乏与致病细菌编码的基因和基因网络的富集增加有关,这些基因和基因网络直接或间接地与内在抗生素耐药性相关。细菌氧化应激与这些小鼠的内在抗生素耐药性有关。该分析揭示了肠道微生物组的多王国改变,这是多种微量营养素缺乏症共同发生的结果,并对抗生素耐药性产生影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


