Above- and belowground fungal biodiversity of Populus trees on a continental scale
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Understanding drivers of terrestrial fungal communities over large scales is an important challenge for predicting the fate of ecosystems under climate change and providing critical ecological context for bioengineering plant–microbe interactions in model systems. We conducted an extensive molecular and microscopy field study across the contiguous United States measuring natural variation in the Populus fungal microbiome among tree species, plant niche compartments and key symbionts. Our results show clear biodiversity hotspots and regional endemism of Populus-associated fungal communities explained by a combination of climate, soil and geographic factors. Modelling climate change impacts showed a deterioration of Populus mycorrhizal associations and an increase in potentially pathogenic foliar endophyte diversity and prevalence. Geographic differences among these symbiont groups in their sensitivity to environmental change are likely to influence broader forest health and ecosystem function. This dataset provides an above- and belowground atlas of Populus fungal biodiversity at a continental scale. This Resource defines fungal diversity of Populus trees and associations with climate variables on a continental scale.
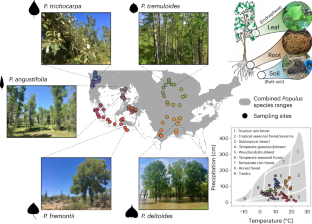
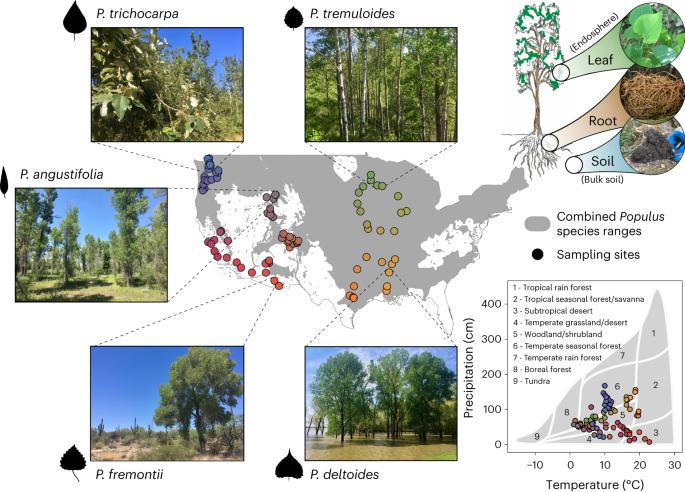
大陆尺度上杨树地上、地下真菌的生物多样性。
了解大规模陆地真菌群落的驱动因素是预测气候变化下生态系统命运的重要挑战,并为模型系统中生物工程植物-微生物相互作用提供关键的生态环境。我们在美国各地进行了广泛的分子和显微镜实地研究,测量了杨树真菌微生物组在树种、植物生态位区室和关键共生体之间的自然变化。研究结果表明,气候、土壤和地理因素共同作用可解释白杨相关真菌群落存在明显的生物多样性热点和区域特有性。气候变化影响模型显示,杨树菌根关联恶化,潜在致病性叶面内生菌多样性和流行率增加。这些共生类群对环境变化敏感性的地理差异可能会影响更广泛的森林健康和生态系统功能。该数据集提供了大陆尺度上杨树真菌生物多样性的地上和地下地图集。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


