Catalytic activation of peroxodisulfate using shape-controlled cerium-manganese composite oxide for phenol degradation: Kinetics and degradation pathway investigation
Abstract
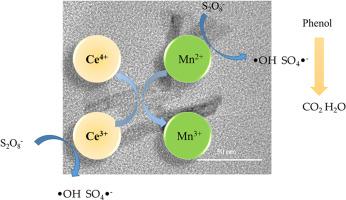
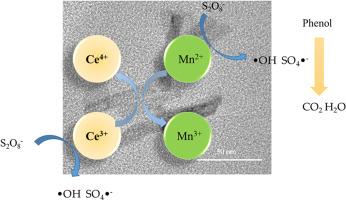
Phenol-containing wastewater is typical organic wastewater, and its treatment is arduous. An advanced method to treat this type of wastewater is persulfate activation. Environmentally friendly cerium-manganese composite oxide materials were synthesized by hydrothermal method and applied to the phenol degradation process. Various ratios of cerium and manganese, as well as the amount of sodium hydroxide, were investigated. The solid solutions of cerium and manganese were formed and confirmed by X-ray diffraction (XRD) and transmission electron microscopy (TEM). H2-temperature programmed reduction (H2-TPR) and X-ray photoelectron spectroscopy (XPS) were utilized to analyze the synergistic effect of cerium and manganese. It is found that there is a transformation between Ce4+/Ce3+ and Mn2+/Mn3+, which makes the material more trivalent manganese and thereby increases the catalytic activity. The effect of materials in catalyzing phenol degradation by peroxodisulfate (PDS) under various preparation conditions is discussed and high-efficiency removal of phenol can be achieved and the removal rate at 180 min is close to 100%. The kinetic of this process was investigated and activation energy of phenol degradation is 62.35 kJ/mol. The degradation pathway of phenol was studied and it is found that PDS can be activated by low metal ions and the ·OH and SO4·– radicals play crucial roles according to the quenching experiments.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


