A facile route for highly enantioenriched six-membered 1,4-N,N- and N,O-heterocycles from L-serinate-derived α-bromoacetates
IF 1.7
4区 化学
引用次数: 0
Abstract
Preparation of highly enantioenriched six-membered 1,4-N,N- and N,O-heterocycles has been developed through [4 + 2] heteroannulation of α-bromoacetates with 1,4-binucleophiles. Two consecutive substitutions of highly diastereoenriched L-serinate-derived α-bromoacetates provide convenient access to a wide range of C-aryl/aliphatic substituted 1,4-N,N- and N,O-heterocycles with up to 99:1 er.
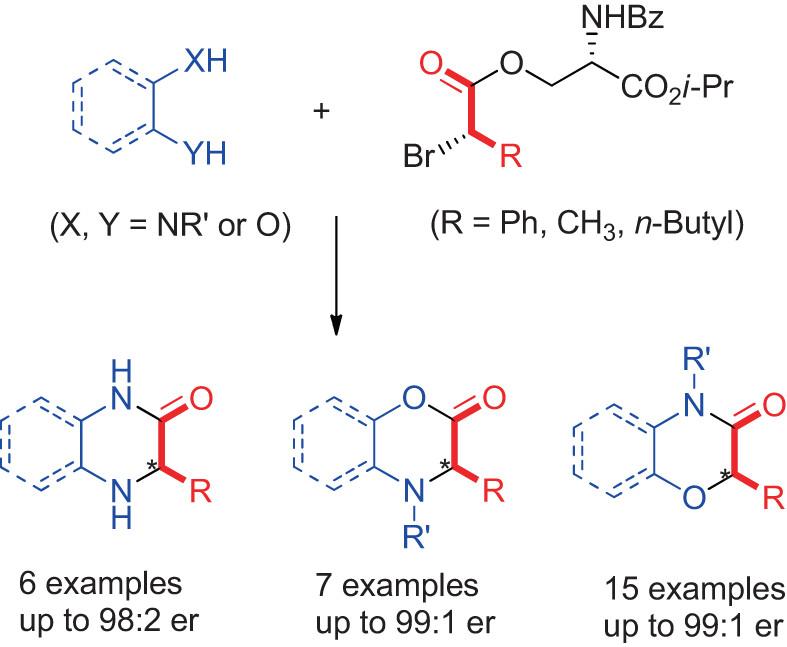
从L-丝氨酸衍生的α-溴乙酸酯制备高对映体富集的六元 1,4-N,N-和 N,O-杂环的简便路线
通过α-溴乙酸酯与 1,4-亲核物的[4 + 2]异嵌合,制备出了高度对映富集的六元 1,4-N,N-和 N,O- 异环。通过连续两次取代高度非对映富集的 L-丝氨酸衍生的 α-溴乙酸酯,可以方便地获得范围广泛的 C-芳基/脂肪族取代的 1,4-N,N-和 N,O- 异环,其比例高达 99:1。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bulletin of the Korean Chemical Society
Chemistry-General Chemistry
自引率
23.50%
发文量
182
期刊介绍:
The Bulletin of the Korean Chemical Society is an official research journal of the Korean Chemical Society. It was founded in 1980 and reaches out to the chemical community worldwide. It is strictly peer-reviewed and welcomes Accounts, Communications, Articles, and Notes written in English. The scope of the journal covers all major areas of chemistry: analytical chemistry, electrochemistry, industrial chemistry, inorganic chemistry, life-science chemistry, macromolecular chemistry, organic synthesis, non-synthetic organic chemistry, physical chemistry, and materials chemistry.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


