Solvent-controlled stereodivergent synthesis of E- and Z-enamines via metal-free formal C(sp2)-H amination of α-substituted styrenes
引用次数: 0
Abstract
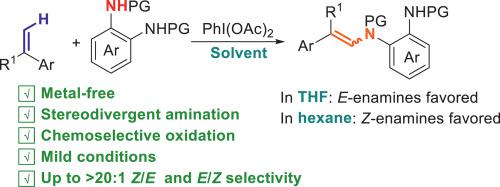
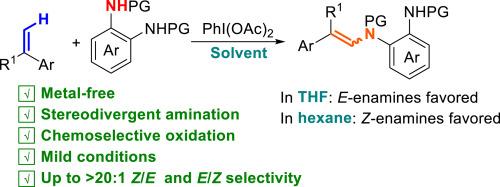
C(sp2)-H amination represents an attractive approach for the synthesis of enamines, which is intrinsically associated with the challenge of controlling of stereochemistry and primarily relying on transition-metal catalysis. Herein, a metal-free C(sp2)-H amination of α-substituted styrenes has been achieved, leading to stereodivergent formation of both E- and Z-enamines in 50 %–97 % yield under mild conditions by using PhI(OAc)2 as a green oxidant and ortho-phenylenediamines as nitrogen source. Interestingly, the Z/E selectivity can be controlled readily by switching the reaction medium. E-isomers were formed preferentially in THF, whereas n-hexane favored the formation of Z-isomers. Preliminary mechanistic studies suggested that in situ formed ortho-benzoquinone diimides are the key intermediates, and there is a correlation between solvent polarity and stereoselectivity. This study enriches the chemical repertoire of ortho-benzoquinone diimides particularly with respect to sustainable amination.
通过α-取代苯乙烯的无金属C(sp2)- h胺化,溶剂控制立体发散合成E-和z -胺
C(sp2)-H胺化是一种有吸引力的胺合成方法,它与立体化学控制的挑战和主要依赖过渡金属催化有着内在的联系。在温和条件下,以PhI(OAc)2为绿色氧化剂,邻苯二胺为氮源,实现了α-取代苯乙烯的无金属C(sp2)- h胺化反应,生成了E-和z -胺,产率为50% - 97%。有趣的是,通过切换反应介质,可以很容易地控制Z/E选择性。在THF中,e -异构体优先形成,而正己烷则有利于z -异构体的形成。初步的机理研究表明,原位形成的邻苯醌二亚胺是关键的中间体,溶剂极性与立体选择性之间存在相关性。这项研究丰富了对苯醌二亚胺的化学库,特别是在可持续胺化方面。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


