Development of Simple HPLC-UV Method for the Simultaneous Determination of Repaglinide, Dexamethasone, and Remdesivir, and its Application to Synthetic Mixture and Human Plasma
IF 1.5
4区 医学
Q4 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Background:: The onset of the COVID-19 pandemic caused numerous difficulties in the treatment of cardiovascular diseases and diabetes mellitus. A persistent risk of developing severe complications and increased mortality from the COVID-19 infection has been reported. In the clinical studies, patients receiving remdesivir and dexamethasone as COVID-19 combination therapy simultaneously with some type II diabetes therapeutic regimens had been reported to have a considerably better state and recover faster. Unfortunately, there is not enough information on the combination of meglitinides, remdesivir, and dexamethasone, and therefore, careful monitoring of the patients' everyday health condition is needed. Objectives:: The present study aimed to describe a high-performance liquid chromatographic method for the determination of repaglinide, dexamethasone, and remdesivir in laboratoryprepared mixtures and human plasma by UV detection. Methods:: Isocratic elution of the mobile phase (consisting of 0.1% trifluoroacetic acid in water and acetonitrile in the ratio 70:30 v/v) was set at a flow rate of 1.0 ml/min, and the developed analytical procedure has been found to be fast and simple. Chromatographic determination was performed on a Purospher® RP – 18 column at room temperature and a UV detector was set at 235 nm. result: The developed method was validated for linearity in the range 2-32 μg/ml. Calibration curves were linear over the selected range with correlation coefficients (R2) greater than 0.996. The coefficients of variation for intraday and interday assay were <2% and the recovery percentages from plasma ranged from 93.83 to 106.49%. Conclusion:: The developed effective and specific method can be applied in routine quality control and clinical laboratory practice.
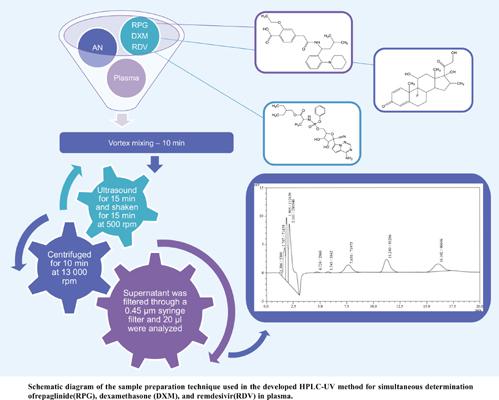
高效液相色谱-紫外同时测定瑞格列奈、地塞米松和瑞德西韦的方法建立及其在复方制剂和人血浆中的应用
背景:COVID-19大流行的爆发给心血管疾病和糖尿病的治疗带来了许多困难。据报道,COVID-19感染存在发生严重并发症和死亡率增加的持续风险。在临床研究中,一些2型糖尿病治疗方案同时接受瑞德西韦和地塞米松联合治疗的患者状态明显更好,恢复更快。不幸的是,关于美格列尼特、瑞德西韦和地塞米松联合使用的信息还不够,因此,需要仔细监测患者的日常健康状况。目的:建立高效液相色谱法测定实验室配制合剂和人血浆中瑞格列奈、地塞米松和瑞德西韦的紫外检测法。方法:流动相(0.1%三氟乙酸水溶液与乙腈按70:30 v/v的比例组成)以1.0 ml/min的流速等压洗脱,所建立的分析方法快速、简便。色谱柱为Purospher®RP - 18,室温,紫外检测器为235 nm。结果:方法在2 ~ 32 μg/ml范围内线性良好。在选择的范围内,校准曲线呈线性,相关系数(R2)大于0.996。日内、日间测定的变异系数为2%,血浆回收率为93.83 ~ 106.49%。结论:所建立的方法有效、特异,可应用于日常质量控制和临床实验室实践。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.50
自引率
0.00%
发文量
85
审稿时长
3 months
期刊介绍:
Aims & Scope
Current Pharmaceutical Analysis publishes expert reviews and original research articles on all the most recent advances in pharmaceutical and biomedical analysis. All aspects of the field are represented including drug analysis, analytical methodology and instrumentation. The journal is essential to all involved in pharmaceutical, biochemical and clinical analysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


