Development of a more efficient catalyst for the redox-neutral organocatalytic mitsunobu reaction by DFT-guided catalyst design
引用次数: 0
Abstract
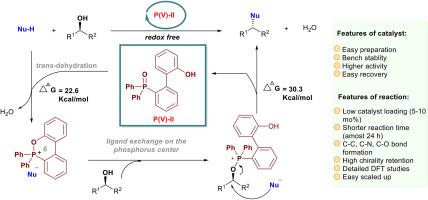
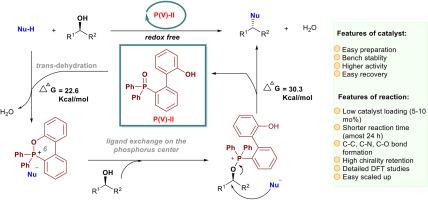
The study of a Mitsunobu reaction is an important topic. Denton and co-workers first reported a novel (2-hydroxybenzyl)diphenylphosphine oxide for realizing the catalytic Mitsunobu reaction via a five-membered phosphonium species. However, it is still worth investigating how to improve catalysts with higher efficiency. Guided by computational and experimental studies, we designed a new type of biphenyl-based phosphine oxide that would form a six-membered phosphonium species as a key intermediate to trigger the catalytic Mitsunobu reaction with a lower barrier of the rate-determining step (30.3 kcal/mol). DFT calculations revealed that only trans dehydration was participated in our catalytic progress and a strong π-π interaction and small spatial constraint of TS-V were crucial for high behavior. This readily accessible, highly stable, easily recyclable and efficient catalyst would boost the catalytic Mitsunobu reaction.
采用dft导向设计一种更高效的氧化还原-中性有机催化mitsunobu反应催化剂
光信反应的研究是一个重要的课题。Denton及其同事首次报道了一种新的(2-羟基苄基)二苯基氧化膦通过五元磷实现催化Mitsunobu反应。然而,如何提高催化剂的效率仍值得进一步研究。在计算和实验研究的指导下,我们设计了一种新型的基于联苯的氧化膦,它可以形成六元磷,作为催化Mitsunobu反应的关键中间体,具有较低的定速步垒(30.3 kcal/mol)。DFT计算表明,催化过程只涉及反式脱水,强π-π相互作用和TS-V的小空间约束是高行为的关键。这种易于获取、高度稳定、易于回收和高效的催化剂将促进催化光信反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


