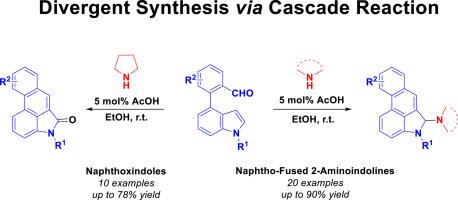
Divergent synthesis of new naphtho-fused 2-aminoindolines and naphthoxindoles based on straightforward construction of phenanthrene
引用次数: 0
Abstract
An environmentally friendly, highly atom-economical and operationally simple approach toward the synthesis of naphtho-fused 2-aminoindolines and naphthoxindoles starting from biaryl aldehydes and secondary amines has been developed. This notable methodology integrates consecutive intramolecular Mannich-type cyclization triggered by the dearomatization of indole with subsequent aromatization via β-elimination and then amination or oxidation at the C2-position of the indole nucleus. The secondary amine-controlled divergent protocol together with an easy product isolation process provides a practical route for the first time to access naphtho-fused 2-substituted indoline.

基于直接构造菲的新型萘融合2-氨基吲哚和萘醌的发散合成
以联芳醛和仲胺为原料,开发了一种环境友好、原子经济性高、操作简单的合成萘融合2-氨基吲哚和萘辛哚的方法。这种引人注目的方法整合了由吲哚去芳构化引发的连续分子内曼尼奇型环化,随后通过β消除芳构化,然后在吲哚核的c2位置进行胺化或氧化。仲胺控制的发散方案和简单的产物分离工艺首次为获得萘- 2-取代吲哚提供了一条实用的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


