Volumetric and Dielectric Properties in Liquid Phase of Dimethyl Ether–Ethanol at (293.2–313.2) K and 1.00 MPa
Abstract
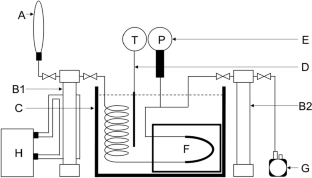
Density and dielectric spectra have been measured in the liquid phase of dimethyl ether (DME)–ethanol by an oscillating U-tube densimeter and a complex dielectric spectrometer under 1.00 MPa. The density was measured at 303.15 and 313.15 K and converted to the excess molar volume. The excess molar volumes were no smaller than − 0.880 and − 0.909 cm3·mol–1 at 303.15 and 313.15 K, respectively. The mole fraction dependence can be correlated with the Redlich–Kister equation, whose minimum was found to be around 0.58 for the mole fraction of DME. The dielectric constant and the dielectric relaxation time were evaluated from the complex dielectric spectra in the frequency range from 0.5 to 18 GHz at (293.2–313.2) K and 1.00 MPa. The dielectric constants and the relaxation time were decreased with the mole fraction of DME, and the latter tended to be around 25 ps in the mole fraction range higher than 0.6. The logarithmic dielectric constants can be correlated with a similar function to the Hildebrand equation with the volume fraction. The effective Kirkwood g-factor was evaluated at 293.2–313.2 K and 1.00 MPa. The g-factors were given by two linear functions crossed around 0.6 for the mole fraction of DME. Considering an atomic composition (C2H6O), the molecular sizes are not so different for DME and ethanol. Then, the solution structure was thought to be microscopically changed around 0.6 for the mole fraction of DME. The mole fraction will be utilized to switch the solubility to extract amphipathic compounds.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


